Acetone, CH3–(C=0) –CH3, and urea, NH2–(C=0)–NH2, have similar chemical structures. (a) Compare the possible intermolecular forces for acetone with those for urea. (b) Compare the intermolecular forces between phycocyanin and acetone with those between phycocyanin and water. Briefly explain how acetone disrupts the folding in phycocyanin. (c) Compare the intermolecular forces between phycocyanin and urea with those between phycocyanin and water. Briefly explain how urea disrupts the folding in phycocyanin. (d) Compare your experimental observation on the disruption of phycocyanin folding in the presence of acetone with that in the presence of urea. Briefly explain whether your comparison is consistent with the properties of acetone and urea.
Acetone, CH3–(C=0) –CH3, and urea, NH2–(C=0)–NH2, have similar chemical structures.
(a) Compare the possible intermolecular forces for acetone with those for urea.
(b) Compare the intermolecular forces between phycocyanin and acetone with those between phycocyanin and water. Briefly explain how acetone disrupts the folding in phycocyanin.
(c) Compare the intermolecular forces between phycocyanin and urea with those between phycocyanin and water. Briefly explain how urea disrupts the folding in phycocyanin.
(d) Compare your experimental observation on the disruption of phycocyanin folding in the presence of acetone with that in the presence of urea. Briefly explain whether your comparison is consistent with the properties of acetone and urea.
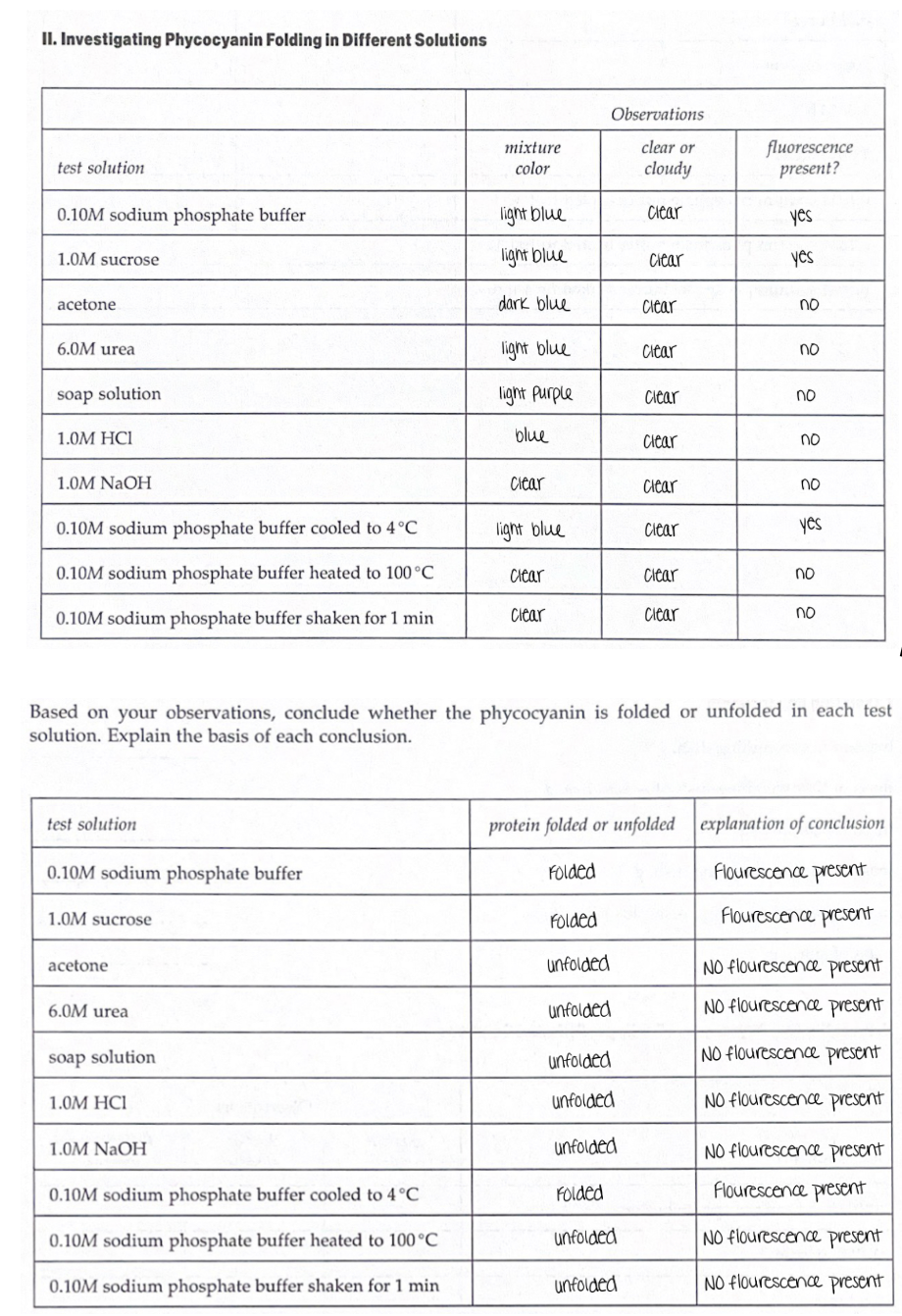
Acetone, CH3–(C=0) –CH3 - it is an organic solvents which unfolds the protein by interfering the mutual attraction between the non-polar groups. At low temperature, proteins are insoluble in acetone while most of the small molecules are soluble. Thus, protein precipitation using acetone can remove the buffer contaminant and eventually can be used to concentrate protein in its pellet. This unfolded precipitated protein can be redissolved in other solvents but solubilized precipitated protein cannot be a folded form.
Urea, NH2–(C=0)–NH2 - it is an organic compound, which unfolds the protein by shifting the equilibrium from the native folded state of protein into denatured state. It works by making the protein-protein contact less stable than protein-urea contact.
Phycocyanin - is a pigment protein complex which belongs to light harvesting phycobiliprotein family. Phycobiliprotein is heterodimers - consist of two - alpha and beta subunits which contains a protein backbone to which 1-2 linear tetrapyrrole chromophores are covalently attached. Phycocyanin is a light blue color pigment phycobiliprotein found in blue green algae. Phycocyanin emits fluorescence at about 650 nm.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps


