Activity 3. The AT:M Family Objectives: 1. Describe the atom and its subatomic particles. 2. Draw an atom and label its parts. Direction: Part 1. Read the basic information about the atom and answer the questions that follow. The term "atom" came from a Greek word "atomos" meaning "indivisible". Today, an atom is defined as the smallest particle that makes up matter. For decades, scientists have been gathering evidence about its structure. Their studies led to the conclusion that atoms are mostly empty space and they have three major subatomic particles. First, the electrons, the negative part of the atom, travel in random paths around its central part known as the nucleus. The nucleus contains the second and third parts, the proton and neutron. The protons and neutrons are both small but massive and their masses are nearly equal. The proton is positively charged, while the neutron has no charge. Protons and neutrons contribute to the mass of the atom. Protons and neutrons are called nucleons because they are found inside the nucleus. The table below shows some of the properties of the three subatomic particles. Subatomic Charge Mass, grams Location in the atom Particle (symbol) Electrons (e') Protons (p) Neutrons (nº) Negative Positive 9.109 X 10 •28 Outside the nucleus 1.672 X 10 -24 Inside the nucleus zero 1.675 X 10 24 Inside the nucleus Guide Questions: 1. What is an atom? 2-4. What are the three major subatomic particles of the atom and their charges? 5. Which subatomic particle is the lightest? 6. Which subatomic particle has no charge? 7. Where can you find the electrons? 8. What is the collective term for protons and neutrons? PART 2. Draw an atom with 2 protons, 2 electrons and 2 neutrons inside the box. Color and label its parts. ATOMIC MODEL SCORING RUBRICS Criteria Atomic model is correctly drawn (2 points) Parts are Score correctly labeled |(2points) Drawing is colored (2 points) Total Score
Activity 3. The AT:M Family Objectives: 1. Describe the atom and its subatomic particles. 2. Draw an atom and label its parts. Direction: Part 1. Read the basic information about the atom and answer the questions that follow. The term "atom" came from a Greek word "atomos" meaning "indivisible". Today, an atom is defined as the smallest particle that makes up matter. For decades, scientists have been gathering evidence about its structure. Their studies led to the conclusion that atoms are mostly empty space and they have three major subatomic particles. First, the electrons, the negative part of the atom, travel in random paths around its central part known as the nucleus. The nucleus contains the second and third parts, the proton and neutron. The protons and neutrons are both small but massive and their masses are nearly equal. The proton is positively charged, while the neutron has no charge. Protons and neutrons contribute to the mass of the atom. Protons and neutrons are called nucleons because they are found inside the nucleus. The table below shows some of the properties of the three subatomic particles. Subatomic Charge Mass, grams Location in the atom Particle (symbol) Electrons (e') Protons (p) Neutrons (nº) Negative Positive 9.109 X 10 •28 Outside the nucleus 1.672 X 10 -24 Inside the nucleus zero 1.675 X 10 24 Inside the nucleus Guide Questions: 1. What is an atom? 2-4. What are the three major subatomic particles of the atom and their charges? 5. Which subatomic particle is the lightest? 6. Which subatomic particle has no charge? 7. Where can you find the electrons? 8. What is the collective term for protons and neutrons? PART 2. Draw an atom with 2 protons, 2 electrons and 2 neutrons inside the box. Color and label its parts. ATOMIC MODEL SCORING RUBRICS Criteria Atomic model is correctly drawn (2 points) Parts are Score correctly labeled |(2points) Drawing is colored (2 points) Total Score
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 10PS: In 1886 Eugene Goldstein observed positively charged particles moving in the opposite direction to...
Related questions
Question
answer all please thankyou
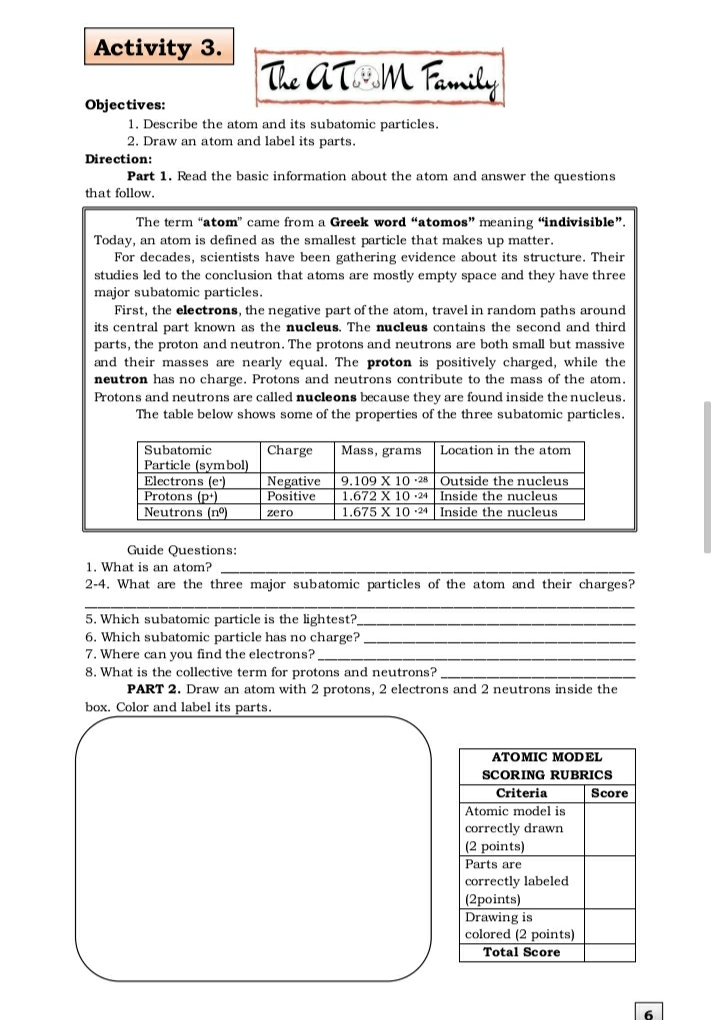
Transcribed Image Text:Activity 3.
The ATM Family
Objectives:
1. Describe the atom and its subatomic particles.
2. Draw an atom and label its parts.
Direction:
Part 1. Read the basic information about the atom and answer the questions
that follow.
The term "atom" came from a Greek word "atomos" meaning "indivisible".
Today, an atom is defined as the smallest particle that makes up matter.
For decades, scientists have been gathering evidence about its structure. Their
studies led to the conclusion that atoms are mostly empty space and they have three
major subatomic particles.
First, the electrons, the negative part of the atom, travel in random paths around
its central part known as the nucleus. The nucleus contains the second and third
parts, the proton and neutron. The protons and neutrons are both small but massive
and their masses are nearly equal. The proton is positively charged, while the
neutron has no charge. Protons and neutrons contribute to the mass of the atom.
Protons and neutrons are called nucleons because they are found inside the nucleus.
The table below shows some of the properties of the three subatomic particles.
Subatomic
Particle (symbol)
Electrons (e)
Protons (p+)
Neutrons (nº)
Charge
Mass, grams
Location in the atom
Negative
Positive
9.109 X 10 -28 Outside the nucleus
1.672 X 10 •24 Inside the nucleus
1.675 X 10 -24 Inside the nucleus
zero
Guide Questions:
1. What is an atom?
2-4. What are the three major subatomic particles of the atom and their charges?
5. Which subatomic particle is the lightest?.
6. Which subatomic particle has no charge?
7. Where can you find the electrons?
8. What is the collective term for protons and neutrons?
PART 2. Draw an atom with 2 protons, 2 electrons and
neutrons inside the
box. Color and label its parts.
ATOMIC MODEL
SCORING RUBRICS
Criteria
Atomic model is
Score
correctly drawn
(2 points)
Parts are
correctly labeled
(2points)
Drawing is
colored (2 points)
Total Score
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning