Activity I Directions: Study the table below. Give the complete set of quantum numbers that describe the following elements. Principal quantum number (n) Azimuthal quantum number (8) Magnetic quantum number (m2) Spin quantum number (m,) Element S Не Na Dy
Activity I Directions: Study the table below. Give the complete set of quantum numbers that describe the following elements. Principal quantum number (n) Azimuthal quantum number (8) Magnetic quantum number (m2) Spin quantum number (m,) Element S Не Na Dy
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter21: Surface Characterization By Spectroscopy And Microscopy
Section: Chapter Questions
Problem 21.11QAP
Related questions
Question
100%
I already splited the questions ,? now pls answer it all i will rate you helpful i dont care about bartleby rules
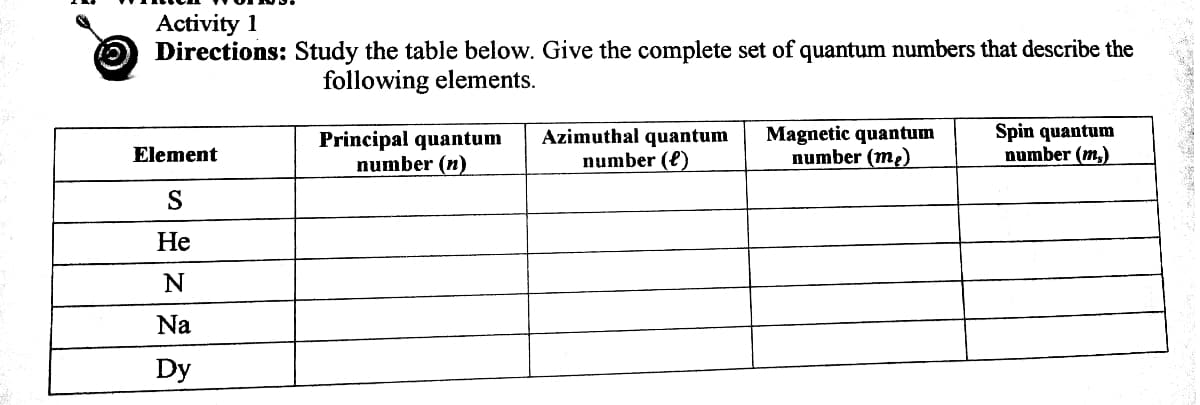
Transcribed Image Text:Activity 1
Directions: Study the table below. Give the complete set of quantum numbers that describe the
following elements.
Principal quantum
number (n)
Azimuthal quantum
number (e)
Magnetic quantum
number (me)
Spin quantum
number (m,)
Element
S
Не
Na
Dy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning