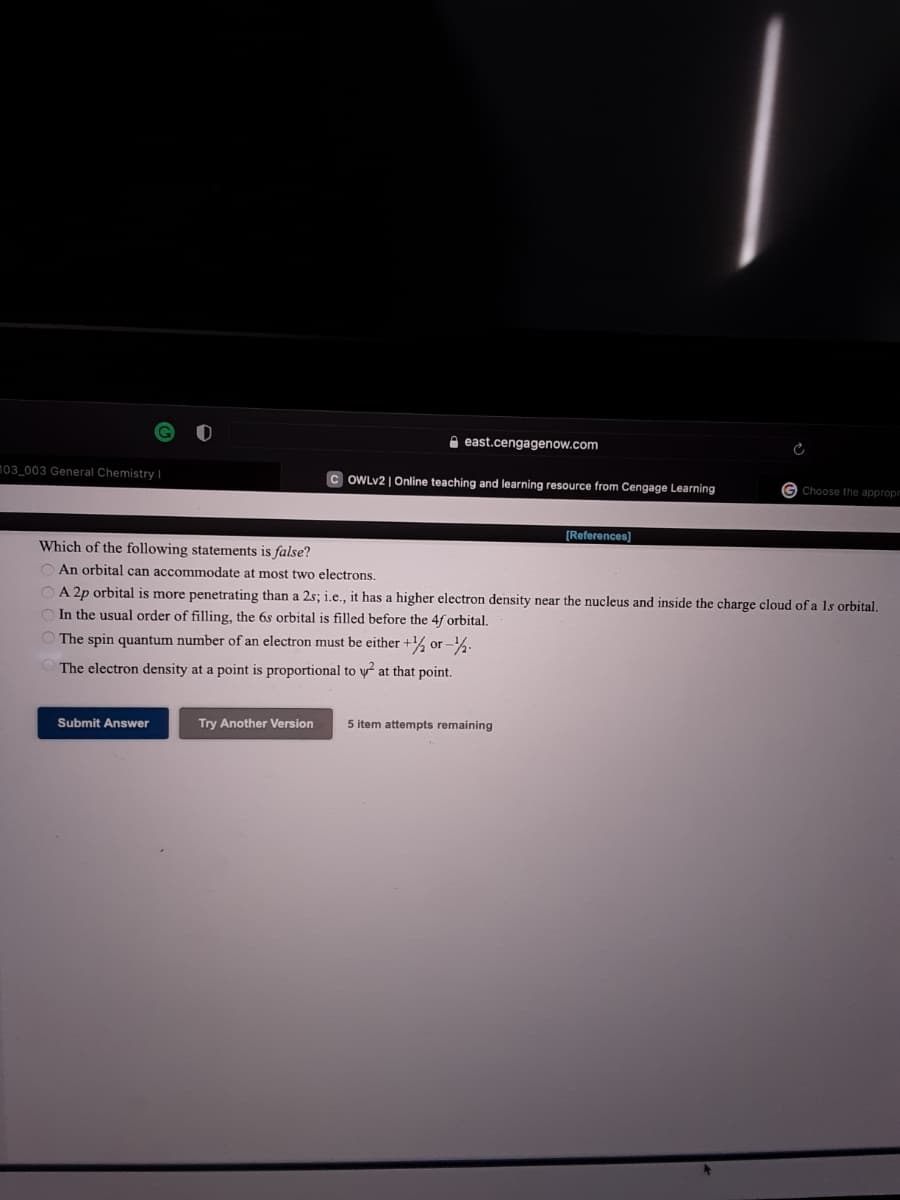
[References] Which of the following statements is false? An orbital can accommodate at most two electrons. A 2p orbital is more penetrating than a 2s; i.e., it has a higher electron density near the nucleus and inside the charge cloud of a Is orbital. In the usual order of filling, the 6s orbital is filled before the 4f orbital. The spin quantum number of an electron must be either +% or -%: The electron density at a point is proportional to y at that point. Submit Answer Try Another Version 5 item attempts remaining
[References] Which of the following statements is false? An orbital can accommodate at most two electrons. A 2p orbital is more penetrating than a 2s; i.e., it has a higher electron density near the nucleus and inside the charge cloud of a Is orbital. In the usual order of filling, the 6s orbital is filled before the 4f orbital. The spin quantum number of an electron must be either +% or -%: The electron density at a point is proportional to y at that point. Submit Answer Try Another Version 5 item attempts remaining
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 30QAP: Consider the following representation of a set of p orbitals for an atom: mg...
Related questions
Question
Transcribed Image Text:e east.cengagenow.com
103 003 General Chemistry I
C OWLV2 | Online teaching and learning resource from Cengage Learning
Choose the appropr
[References)
Which of the following statements is false?
O An orbital can accommodate at most two electrons.
OA 2p orbital is more penetrating than a 2s; i.e., it has a higher electron density near the nucleus and inside the charge cloud of a ls orbital.
O In the usual order of filling, the 6s orbital is filled before the 4f orbital,
The spin quantum number of an electron must be either +% or -%.
The electron density at a point is proportional to y at that point.
Submit Answer
Try Another Version
5 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning