After adding 6M HCl to the unknown solution, the resulting solid is separated from the liquid. Hot water is next added to the solid. Not mixing the solid real well with the hot water could result in false-positive results for which ion(s)?
After adding 6M HCl to the unknown solution, the resulting solid is separated from the liquid. Hot water is next added to the solid. Not mixing the solid real well with the hot water could result in false-positive results for which ion(s)?
Chapter14: Chromatography
Section: Chapter Questions
Problem 1P
Related questions
Question
After adding 6M HCl to the unknown solution, the resulting solid is separated from the liquid. Hot water is next added to the solid. Not mixing the solid real well with the hot water could result in false-positive results for which ion(s)?
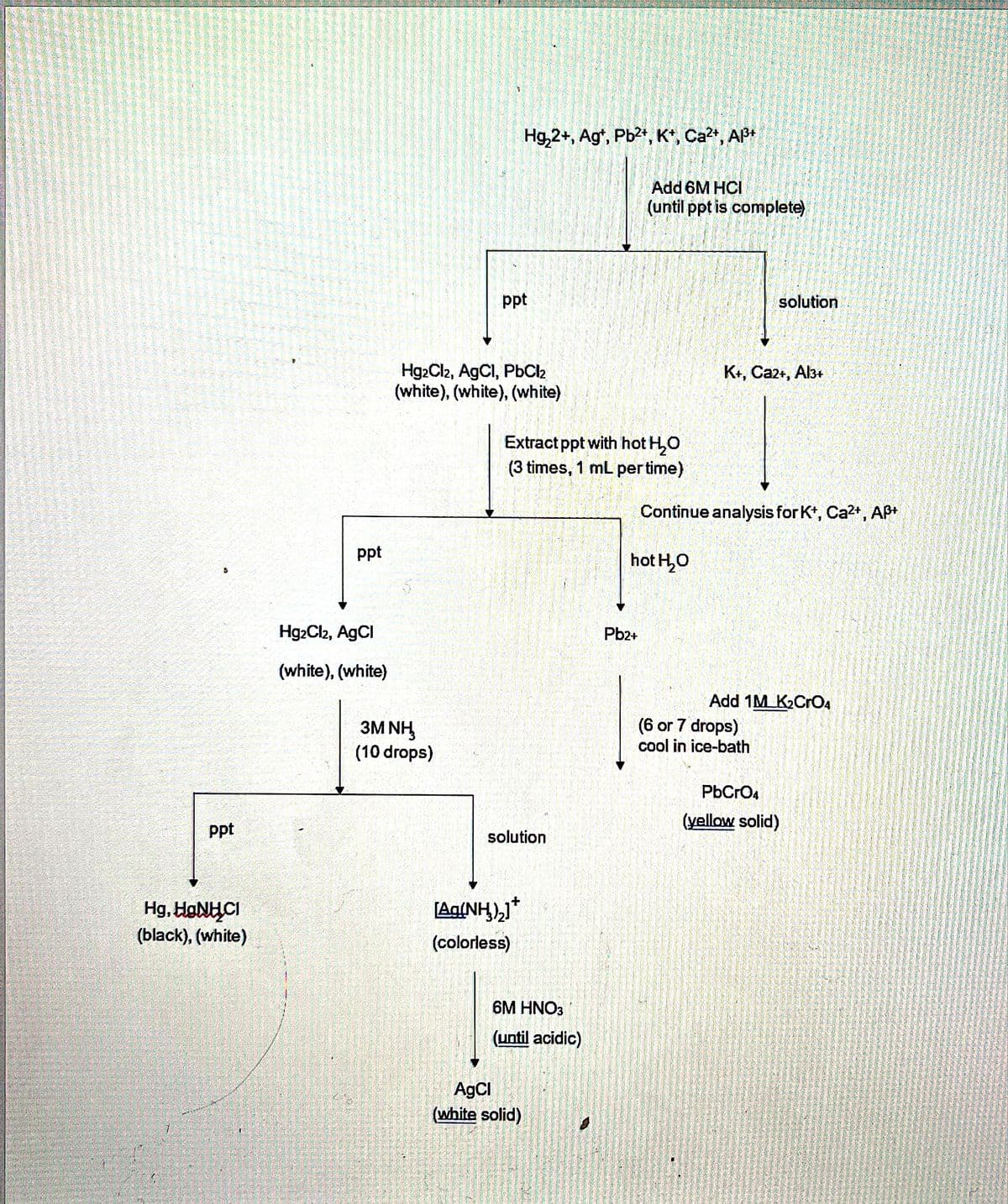
Transcribed Image Text:Hg,2+, Ag*, Pb2*, K*, Ca2", AP+
Add 6M HCI
(until ppt is complete)
ppt
solution
Hg2Cl2, AgCI, PbCl2
(white), (white), (white)
K+, Ca2+, Al3+
Extract ppt with hot H,O
(3 times, 1 mL per time)
Continue analysis for Kt, Ca2+, AP+
ppt
hot H,O
Hg2Cl2, AgCI
Pb2+
(white), (white)
Add 1M K2CrO4
3M NH
(10 drops)
(6 or 7 drops)
cool in ice-bath
PbCrO4
(yellow solid)
ppt
solution
+
[Ag{NH),j*
(black), (white)
(colorless)
6M HNO3
(until acidic)
AgCl
(white solid)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole
