For each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to estimate solubilitles. (If more than one salt on the right can be directly compared, Include all the relevant salts by writing your answer as a string of characters without punctuation, e.g, ABC.) 1. lead hydroxide 2. iron(II) carbonate A. Cus В. ВаСОз C. Cu(OH)2 D. Ag2S Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water. lead hydroxide iron(II) carbonate Ksp Ksp
For each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to estimate solubilitles. (If more than one salt on the right can be directly compared, Include all the relevant salts by writing your answer as a string of characters without punctuation, e.g, ABC.) 1. lead hydroxide 2. iron(II) carbonate A. Cus В. ВаСОз C. Cu(OH)2 D. Ag2S Write the expression for K in terms of the solubility, s, for each salt, when dissolved in water. lead hydroxide iron(II) carbonate Ksp Ksp
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter20: Acidity And Pka Of Phenols
Section: Chapter Questions
Problem 3CTQ
Related questions
Question
100%
Help please
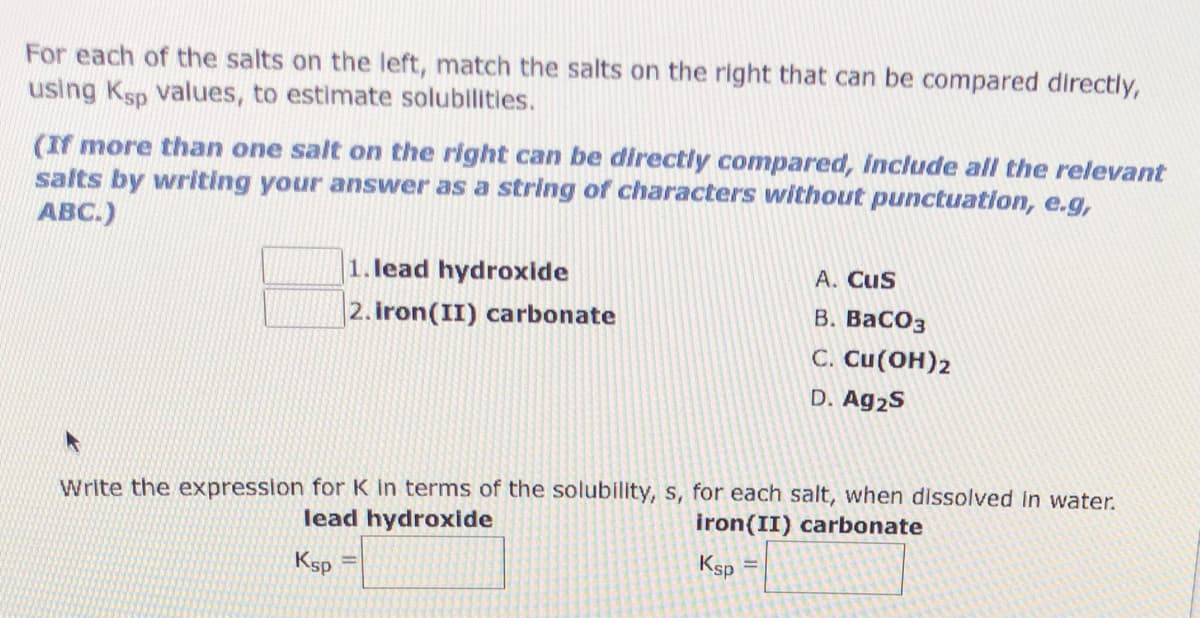
Transcribed Image Text:For each of the salts on the left, match the salts on the right that can be compared directly,
using Ksp values, to estimate solubilities.
(If more than one salt on the right can be directly compared, Include all the relevant
salts by writing your answer as a string of characters without punctuation, e.g,
ABC.)
|1. lead hydroxide
2. iron(II) carbonate
A. Cus
В. ВаСОз
C. Cu(OH)2
D. Ag2S
Write the expression for K in terms of the solubility, s, for each salt, when dissolved In water.
lead hydroxide
iron(II) carbonate
Ksp
Ksp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole