Agent Precipitate Precipitate Ag+ Insoluble Dilute HCI chlorides Insoluble chlorides Pb2+ Dilute HCI The Insoluble low amounts of sulfide ion; by adding an acidic solution of H2S. The Insoluble low amounts of sulfide ion; by adding an acidic solution of H2S. relatively high amounts of II Sulfides in Acidic Medium Cu2+ II Sulfides in Acidic Medium The Insoluble Sulfides in Fe3+ II Basic Medium sulfide ion; adding a basic solution of H2S. relatively high amounts of sulfide ion; Ni2+ II The Insoluble Sulfides in Basic Medium adding a basic solution of H2S. Insoluble Carbonates Insoluble Carbonates The Soluble Group The Soluble Group Ca2+ IV Alkaline Solution Ba2+ IV Alkaline Solution Na+ V None K* V None
Agent Precipitate Precipitate Ag+ Insoluble Dilute HCI chlorides Insoluble chlorides Pb2+ Dilute HCI The Insoluble low amounts of sulfide ion; by adding an acidic solution of H2S. The Insoluble low amounts of sulfide ion; by adding an acidic solution of H2S. relatively high amounts of II Sulfides in Acidic Medium Cu2+ II Sulfides in Acidic Medium The Insoluble Sulfides in Fe3+ II Basic Medium sulfide ion; adding a basic solution of H2S. relatively high amounts of sulfide ion; Ni2+ II The Insoluble Sulfides in Basic Medium adding a basic solution of H2S. Insoluble Carbonates Insoluble Carbonates The Soluble Group The Soluble Group Ca2+ IV Alkaline Solution Ba2+ IV Alkaline Solution Na+ V None K* V None
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.40QAP
Related questions
Question
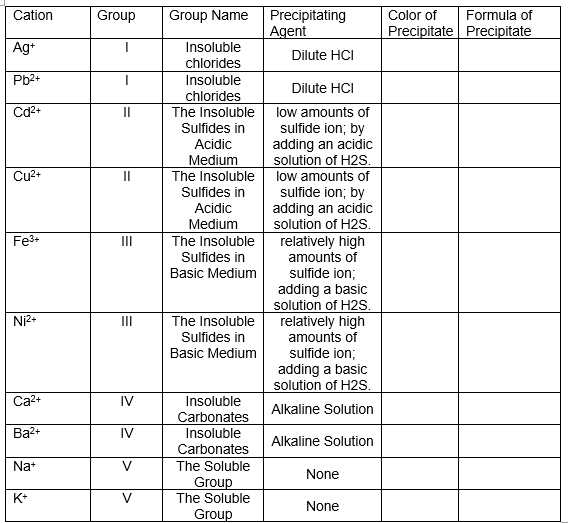
Transcribed Image Text:Group Name
Precipitating
Agent
Cation
Group
Color of
Formula of
Precipitate Precipitate
Ag+
Insoluble
Dilute HCI
chlorides
Pb2+
Insoluble
Dilute HCI
chlorides
The Insoluble low amounts of
sulfide ion; by
adding an acidic
solution of H2S.
The Insoluble low amounts of
sulfide ion; by
adding an acidic
solution of H2S.
relatively high
Cd2+
II
Sulfides in
Acidic
Medium
Cu2+
II
Sulfides in
Acidic
Medium
The Insoluble
Fe3+
II
Sulfides in
amounts of
Basic Medium
sulfide ion;
adding a basic
solution of H2S.
relatively high
Ni2+
II
The Insoluble
Sulfides in
amounts of
Basic Medium
sulfide ion;
adding a basic
solution of H2S.
Ca2+
IV
Insoluble
Alkaline Solution
Carbonates
Insoluble
Ba2+
IV
Alkaline Solution
Carbonates
Na+
V
The Soluble
None
Group
The Soluble
K+
None
Group
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

