TABLE 2.6 Some weak acids and their conjugate bases Acid (Proton Donor) Conjugate Base (Proton Acceptor) pKa Ka (M) HCOOH HCOO- +H+ 3.75 1.78 x 10-4 Formic acid Formate ion CH;COOH Acetic acid CH3CO0- Acetate ion +H+ 4.76 1.74 x 10-5 OH OH +H+ 3.86 1.38 x 10-4 CH,CH-Co0- CH3CH-COOH Lactic acid Lactate ion H3PO4 Phosphoric acid H,PO, Dihydrogen phosphate ion +H+ 2.14 7.24 x 10-3 H,PO, Dihydrogen phosphate ion HPO,?- Monohydrogen phosphate ion +H+ 6.86 1.38 x 10-7 HPO- Monohydrogen phosphate ion PO,- Phosphate ion +H+ 12.4 3.98 x 10-13 H,CO3 Carbonic acid HCO,- Bicarbonate ion +H+ 6.3* 5.1 x 10-7* HCO,- Bicarbonate ion co?- Carbonate ion +H* 10.25 5.62 x 10-11 C6H5OH Phenol +H+ 9.89 1.29 x 10-10 Phenolate ion NH3 Ammonia +H+ 9.25 5.62 x 10-10 Ammonium ion "Apparent pk, and K. values (see text for explanation). 1 1
TABLE 2.6 Some weak acids and their conjugate bases Acid (Proton Donor) Conjugate Base (Proton Acceptor) pKa Ka (M) HCOOH HCOO- +H+ 3.75 1.78 x 10-4 Formic acid Formate ion CH;COOH Acetic acid CH3CO0- Acetate ion +H+ 4.76 1.74 x 10-5 OH OH +H+ 3.86 1.38 x 10-4 CH,CH-Co0- CH3CH-COOH Lactic acid Lactate ion H3PO4 Phosphoric acid H,PO, Dihydrogen phosphate ion +H+ 2.14 7.24 x 10-3 H,PO, Dihydrogen phosphate ion HPO,?- Monohydrogen phosphate ion +H+ 6.86 1.38 x 10-7 HPO- Monohydrogen phosphate ion PO,- Phosphate ion +H+ 12.4 3.98 x 10-13 H,CO3 Carbonic acid HCO,- Bicarbonate ion +H+ 6.3* 5.1 x 10-7* HCO,- Bicarbonate ion co?- Carbonate ion +H* 10.25 5.62 x 10-11 C6H5OH Phenol +H+ 9.89 1.29 x 10-10 Phenolate ion NH3 Ammonia +H+ 9.25 5.62 x 10-10 Ammonium ion "Apparent pk, and K. values (see text for explanation). 1 1
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter15: Solutions Of Acids And Bases
Section: Chapter Questions
Problem 15.23QE
Related questions
Question
A biochemical reaction takes place in a 1.00 ml solution of 0.0250 M
phosphate buffer initially at pH = 7.20 (as shown for pKas of phosphate
species).
(a) Are the concentrations of any of the four possible phosphate species
negligible? If so, identify them and explain your answer.
(b) During the reaction, 3.80 μmol of HCl are produced. Calculate the final
pH of the reaction solution. Assume that the HCl is completely neutralized
by the buffer.
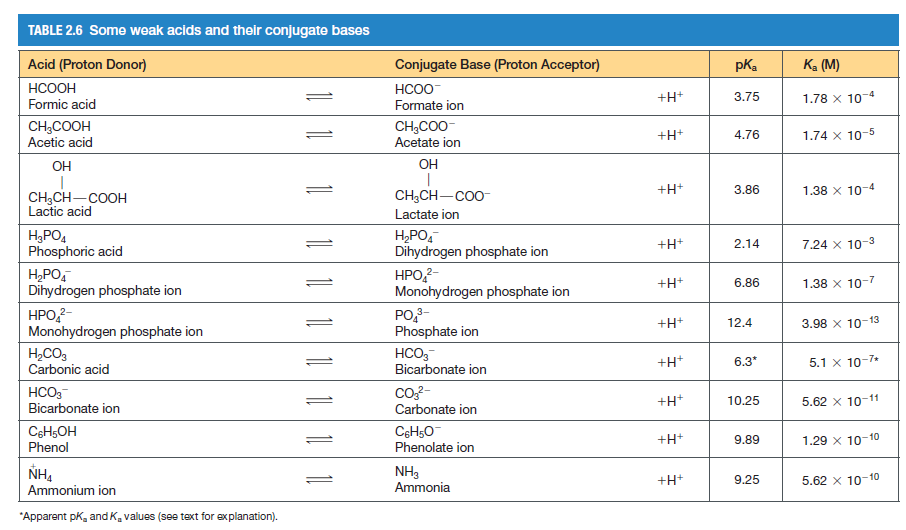
Transcribed Image Text:TABLE 2.6 Some weak acids and their conjugate bases
Acid (Proton Donor)
Conjugate Base (Proton Acceptor)
pKa
Ka (M)
HCOOH
HCOO-
+H+
3.75
1.78 x 10-4
Formic acid
Formate ion
CH;COOH
Acetic acid
CH3CO0-
Acetate ion
+H+
4.76
1.74 x 10-5
OH
OH
+H+
3.86
1.38 x 10-4
CH,CH-Co0-
CH3CH-COOH
Lactic acid
Lactate ion
H3PO4
Phosphoric acid
H,PO,
Dihydrogen phosphate ion
+H+
2.14
7.24 x 10-3
H,PO,
Dihydrogen phosphate ion
HPO,?-
Monohydrogen phosphate ion
+H+
6.86
1.38 x 10-7
HPO-
Monohydrogen phosphate ion
PO,-
Phosphate ion
+H+
12.4
3.98 x 10-13
H,CO3
Carbonic acid
HCO,-
Bicarbonate ion
+H+
6.3*
5.1 x 10-7*
HCO,-
Bicarbonate ion
co?-
Carbonate ion
+H*
10.25
5.62 x 10-11
C6H5OH
Phenol
+H+
9.89
1.29 x 10-10
Phenolate ion
NH3
Ammonia
+H+
9.25
5.62 x 10-10
Ammonium ion
"Apparent pk, and K. values (see text for explanation).
1 1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning