Air (a diatomic ideal gas) at 27.0°C and atmospheric pressure is drawn into a bicycle pump that has a cylinder with an inner diameter of 2.50 cm and length 50.0 cm. The down stroke adiabatically compresses the air, which reaches a gauge pressure of 800 kPa before entering the tire (Fig. P21.26). Determine (a) the volume of the compressed air and (b) the temperature of the compressed air. (c) What If? The pump is made of steel and has an inner wall that is 2.00 mm thick. Assume that 4.00 cm of the cylinder's length is allowed to come to thermal equilibrium with the air. What will be the increase in wall temperature?
Air (a diatomic ideal gas) at 27.0°C and atmospheric pressure is drawn into a bicycle pump that has a cylinder with an inner diameter of 2.50 cm and length 50.0 cm. The down stroke adiabatically compresses the air, which reaches a gauge pressure of 800 kPa before entering the tire (Fig. P21.26). Determine (a) the volume of the compressed air and (b) the temperature of the compressed air. (c) What If? The pump is made of steel and has an inner wall that is 2.00 mm thick. Assume that 4.00 cm of the cylinder's length is allowed to come to thermal equilibrium with the air. What will be the increase in wall temperature?
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter21: Heat And The First Law Of Thermodynamics
Section: Chapter Questions
Problem 54PQ
Related questions
Question
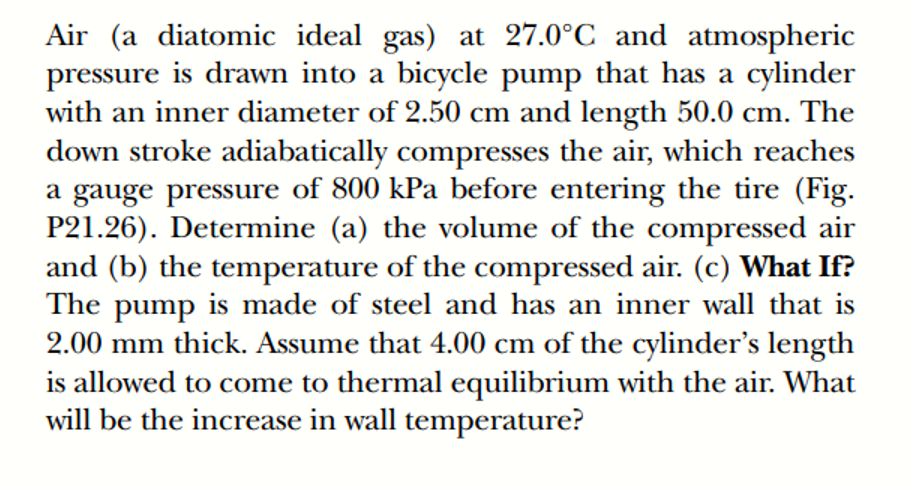
Transcribed Image Text:Air (a diatomic ideal gas) at 27.0°C and atmospheric
pressure is drawn into a bicycle pump that has a cylinder
with an inner diameter of 2.50 cm and length 50.0 cm. The
down stroke adiabatically compresses the air, which reaches
a gauge pressure of 800 kPa before entering the tire (Fig.
P21.26). Determine (a) the volume of the compressed air
and (b) the temperature of the compressed air. (c) What If?
The pump is made of steel and has an inner wall that is
2.00 mm thick. Assume that 4.00 cm of the cylinder's length
is allowed to come to thermal equilibrium with the air. What
will be the increase in wall temperature?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning
