Airbag experiment The simulation link is provided below if needed. It's probably best to use the simulation because the pictures attached won't show everything in full. Simulation link: https://interactives.ck12.org/simulations/chemistry/decomposition-reaction/app/index.html?screen=sandbox&lang=en&referrer=ck12Launcher&backUrl=https://interactives.ck12.org/simulations/chemistry.html Just select ammonium nitrate and then select 1 mole and press play. Record macroscopic observations of the bag and the crash test dummy as well as microscopic observations of the particles within the steering mechanism and the inflated/inflating airbag.
Airbag experiment The simulation link is provided below if needed. It's probably best to use the simulation because the pictures attached won't show everything in full. Simulation link: https://interactives.ck12.org/simulations/chemistry/decomposition-reaction/app/index.html?screen=sandbox&lang=en&referrer=ck12Launcher&backUrl=https://interactives.ck12.org/simulations/chemistry.html Just select ammonium nitrate and then select 1 mole and press play. Record macroscopic observations of the bag and the crash test dummy as well as microscopic observations of the particles within the steering mechanism and the inflated/inflating airbag.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter5: Gases
Section: Chapter Questions
Problem 129E
Related questions
Question
Airbag experiment
The simulation link is provided below if needed. It's probably best to use the simulation because the pictures attached won't show everything in full.
Simulation link:
https://interactives.ck12.org/simulations/chemistry/decomposition-reaction/app/index.html?screen=sandbox&lang=en&referrer=ck12Launcher&backUrl=https://interactives.ck12.org/simulations/chemistry.html
Just select ammonium nitrate and then select 1 mole and press play.
Record macroscopic observations of the bag and the crash test dummy as well as microscopic observations of the particles within the steering mechanism and the inflated/inflating airbag.
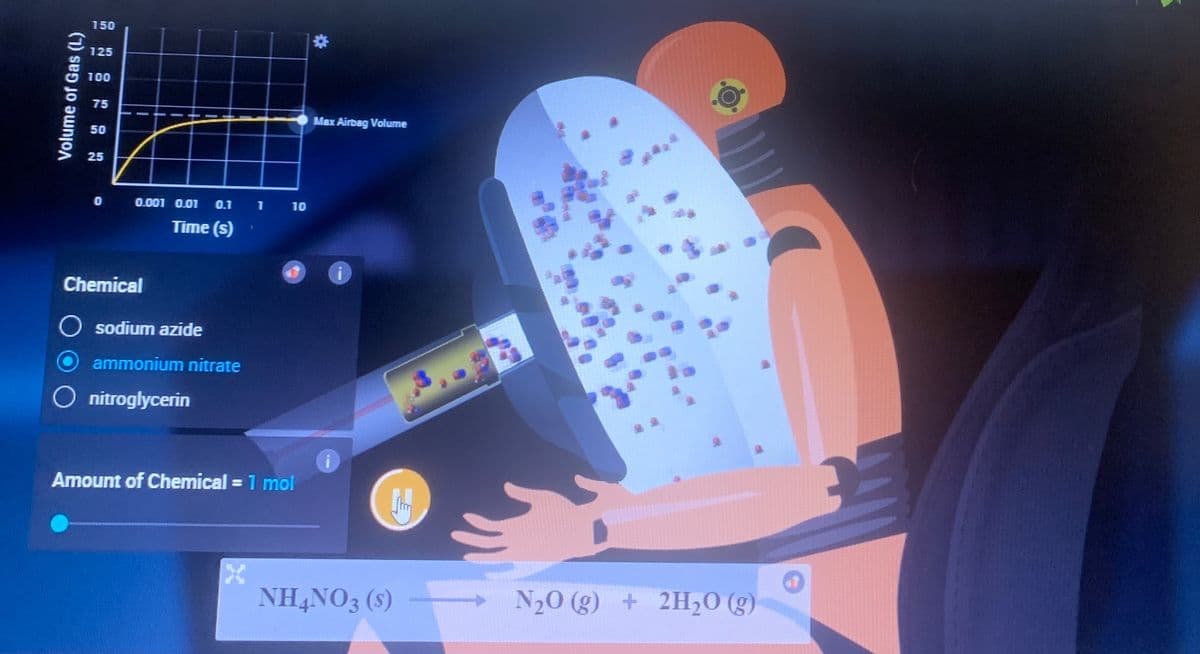
Transcribed Image Text:Volume of Gas (L)
150
125
100
75
50
25
|
0 0.001 0.01 0.1
Time (s)
Chemical
O sodium azide
O ammonium nitrate
O nitroglycerin
1
10
Amount of Chemical = 1 mol
Max Airbag Volume
i
NH4NO3 (s)
N₂O(g) + 2H₂O(g)
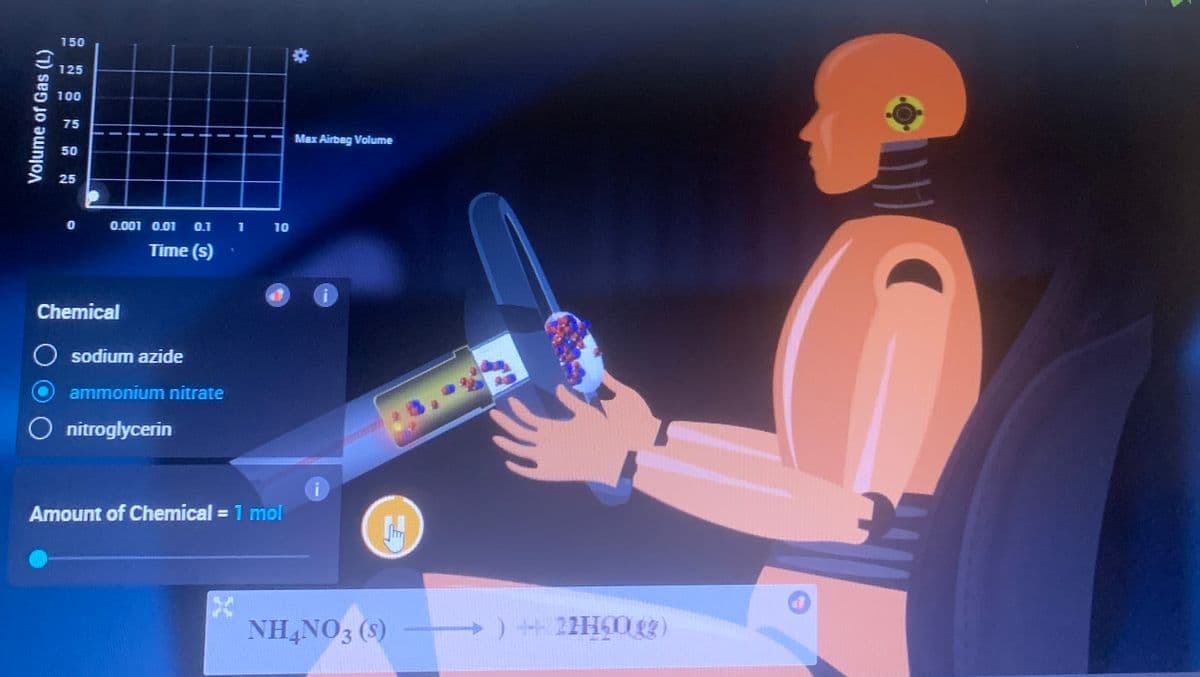
Transcribed Image Text:Volume of Gas (L)
150
125
100
75
50
25
1
I
0 0.001 0.01
Chemical
I
O sodium azide
+
Time (s)
O nitroglycerin
I
T
0.1 1 10
ammonium nitrate
Amount of Chemical = 1 mol
*
Max Airbag Volume
Shm
NH4NO3 (s)
+) 22H60.gg)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
