alage image 1) Based on the model, which conclusion can be made? A) As volume increases, pressure decreases. B) As volume increases, pressure increases. Temp 25°C Pressure = 8 atm Volume increases as temperature increases. Temp. = 25°C Pressure = 2 atm C) D) Density decreases as temperature decreases. 2) Temperature also affects the motion of atoms in a gas. Given what you know about temperature, pressure, and volume, which statement is NOT correct? A) As a gas is heated, the volume increases. B) As a gas is heated, the pressure increases. C) As a gas is heated, the pressure decreases. As a gas is heated, energy increases and molecules D) spread out.
alage image 1) Based on the model, which conclusion can be made? A) As volume increases, pressure decreases. B) As volume increases, pressure increases. Temp 25°C Pressure = 8 atm Volume increases as temperature increases. Temp. = 25°C Pressure = 2 atm C) D) Density decreases as temperature decreases. 2) Temperature also affects the motion of atoms in a gas. Given what you know about temperature, pressure, and volume, which statement is NOT correct? A) As a gas is heated, the volume increases. B) As a gas is heated, the pressure increases. C) As a gas is heated, the pressure decreases. As a gas is heated, energy increases and molecules D) spread out.
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter13: Temperature, Kinetic Theory, And The Gas Laws
Section: Chapter Questions
Problem 68PE: Integrated Concepts If you want to cook in water at 150C, you need a pressure cooker that can...
Related questions
Question
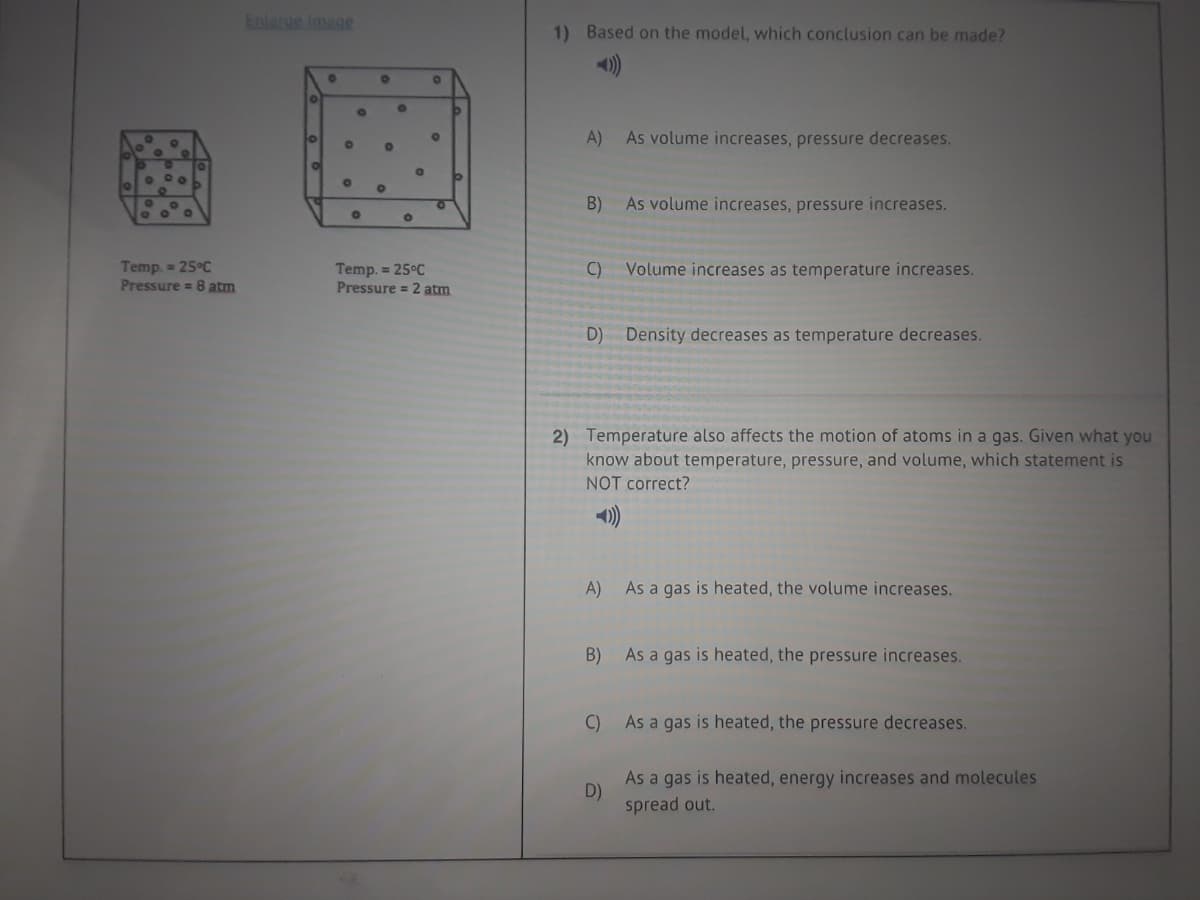
Transcribed Image Text:Enlarge Image
1) Based on the model, which conclusion can be made?
A)
As volume increases, pressure decreases.
01
of
B)
As volume increases, pressure increases.
Temp. = 25°C
Pressure = 8 atm
Volume increases as temperature increases.
Temp. = 25°C
Pressure = 2 atm
C)
D) Density decreases as temperature decreases.
2) Temperature also affects the motion of atoms in a gas. Given what you
know about temperature, pressure, and volume, which statement is
NOT correct?
A)
As a gas is heated, the volume increases.
B)
As a gas is heated, the pressure increases.
C)
As a gas is heated, the pressure decreases.
As a gas is heated, energy increases and molecules
D)
spread out.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill