A container holds a sample of ideal gas in thermal equilibrium, as shown in the figure. (Figure 1) One end of the container is sealed with a piston whose head is perfectly free to move, unless it is locked in place. The walls of the container readily allow the transfer of energy via heat, unless the piston is insulated from its surroundings. Refer to the pV diagram presented to answer the questions below. (Figure 2) In each case, the piston head is initially unlocked and the gas is in equilibrium at the pressure and volume indicated by point 0 on the diagram. Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process? "Lock the piston head in place, and hold the container above a very hot flame." Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process? "Immerse the container into a large water bath at the same temperature, and very slowly push the piston head further into the container." Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process? "Lock the piston head in place and plunge the container into water that is colder than the gas." Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process? "The piston is now insulated from its surroundings. Pull the piston head further out of the container."
A container holds a sample of ideal gas in thermal equilibrium, as shown in the figure. (Figure 1) One end of the container is sealed with a piston whose head is perfectly free to move, unless it is locked in place. The walls of the container readily allow the transfer of energy via heat, unless the piston is insulated from its surroundings.
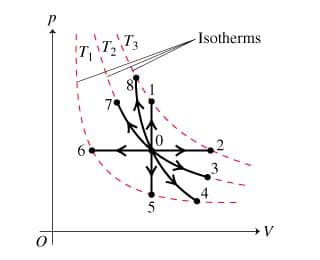
Refer to the pV diagram presented to answer the questions below. (Figure 2) In each case, the piston head is initially unlocked and the gas is in equilibrium at the pressure and volume indicated by point 0 on the diagram.
Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process?
"Lock the piston head in place, and hold the container above a very hot flame."
Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process?
"Immerse the container into a large water bath at the same temperature, and very slowly push the piston head further into the container."
Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process?
"Lock the piston head in place and plunge the container into water that is colder than the gas."
Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process?
"The piston is now insulated from its surroundings. Pull the piston head further out of the container."
Trending now
This is a popular solution!
Step by step
Solved in 2 steps






