Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter5: Chemical Reactions
Section: Chapter Questions
Problem 5.43E: Calculate the number of moles of CO2 generated by the reaction of Exercise 5.42 when 500.g of CaO is...
Related questions
Question
All data is attached,
please help solve the last section and Molarity KMnO4
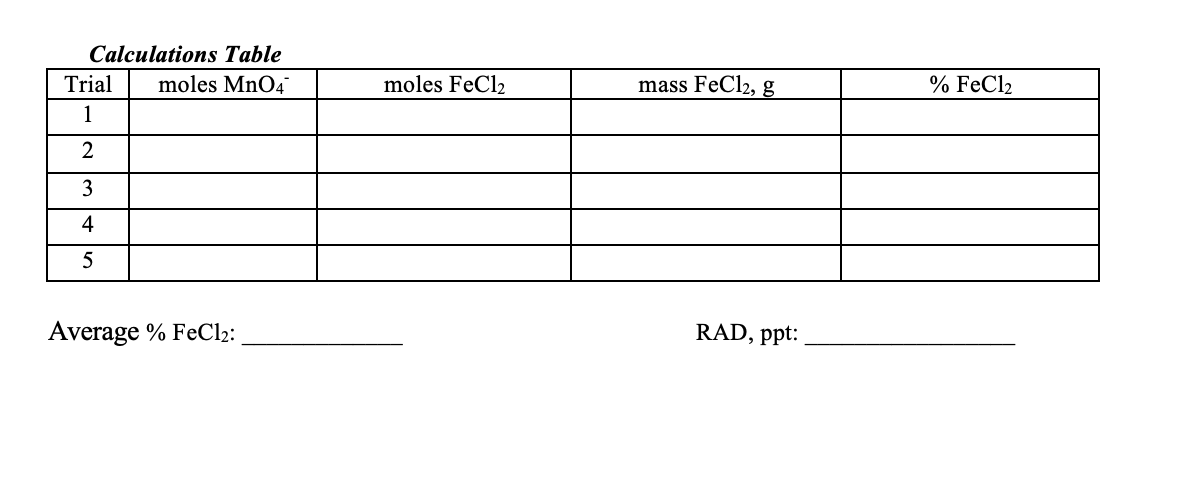
Transcribed Image Text:Calculations Table
Trial
moles MnO4
moles FeCl2
mass FeCl2, g
% FeCl2
1
2
3
4
5
Average % FeCl2:
RAD, ppt:

Transcribed Image Text:Unknovwn #
Molarity KMHO4
Data Table
Trial
mass FeCl2 sample (g)
volume KMN04 (mL)
1
2.0595
25.32
1.9725
24.45
3
1.9811
24.60
4
1.9134
24.03
5
1.9417
24.16
6. Write a balanced net ionic equation for the reaction in acidic solution of FeCl2 and KMNO4 (Fe²* is
oxidized to Fe** and MnO4 is reduced to Mn²*).
Mn0i+8M*+5Fe²+>Mn²*+5F¢3++4H20
Use the Calculations Table below to input the results from the following calculations. Show the
calculations for one trial in the following spaces. You may do the calculations for the other trials on a
separate piece of paper.
7. The moles of MnO4 can be calculated by multiplying the volume of MnO4 required to reach the
endpoint, in L, multiplied by the molarity of the MnO4 solution.
0.0815 mol/L * 25.32 mL * 1 L/1000 mL = 0.002064 moles
8. The moles of FeCl2 can be calculated by using the mole ratio from the balanced equation.
Mn04-+ 8H + 5FE2+ ==> Mn2+ + 5FE3++ 4H2O
this is 1:5 reaction :
moles of FeC12 ==> 0.002064 mole MnO4 * 5 mol FeC12 / 1 mol mole MnO4 = 0.01032
moles
9. The mass of FeCl2 in the sample can be calculated by multiplying the moles of FeCl2 by the
molecular weight of FeCl2.
0.01032 moles* 151.91 g/mol = 1.5674 g
10. The mass % of FeCl2 in the unknown sample can be calculated by dividing the mass of FeCl2 in the
sample by the total mass of the unknown sample, and multiplying by 100. Calculate the average of
the five trials and the RAD of the % FeCl2.
1.5674 g/2.0595 g*100 = 76.1 %
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co