Aluminum reacts with aqueous sodium hydroxide to produce hydrogen gas according to the following equation: 2AI9) + 2NaOH(aq) + 6H,00)—2NaA{ОН), (aq) + 3H2(g) The product gas, H2, is collected over water at a temperature of 20 °C and a pressure of 741 mm Hg. If the wet H2 gas formed occupies a volume of 6.38 L, the number of moles of Al reacted was | |mol. The vapor pressure of water is 17.5 mm Hg at 20 °C.
Aluminum reacts with aqueous sodium hydroxide to produce hydrogen gas according to the following equation: 2AI9) + 2NaOH(aq) + 6H,00)—2NaA{ОН), (aq) + 3H2(g) The product gas, H2, is collected over water at a temperature of 20 °C and a pressure of 741 mm Hg. If the wet H2 gas formed occupies a volume of 6.38 L, the number of moles of Al reacted was | |mol. The vapor pressure of water is 17.5 mm Hg at 20 °C.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.24P
Related questions
Question
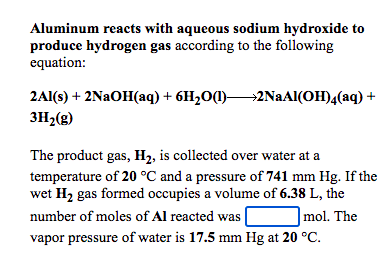
Transcribed Image Text:Aluminum reacts with aqueous sodium hydroxide to
produce hydrogen gas according to the following
equation:
2Al(s) + 2NAOH(aq) + 6H2O(1)2NAAI(OH)4(aq) +
3H2(g)
The product gas, H2, is collected over water at a
temperature of 20 °C and a pressure of 741 mm Hg. If the
wet H2 gas formed occupies a volume of 6.38 L, the
|mol. The
number of moles of Al reacted was |
vapor pressure of water is 17.5 mm Hg at 20 °C.
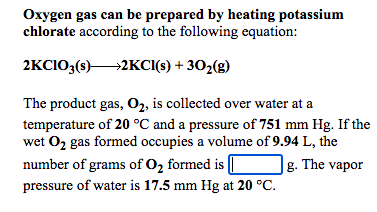
Transcribed Image Text:Oxygen gas can be prepared by heating potassium
chlorate according to the following equation:
2KCIO;(s)2KCI(s) + 302(g)
The product gas, O2, is collected over water at a
temperature of 20 °C and a pressure of 751 mm Hg. If the
wet O2 gas formed occupies a volume of 9.94 L, the
number of grams of O2 formed is [
g. The vapor
pressure of water is 17.5 mm Hg at 20 °C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning