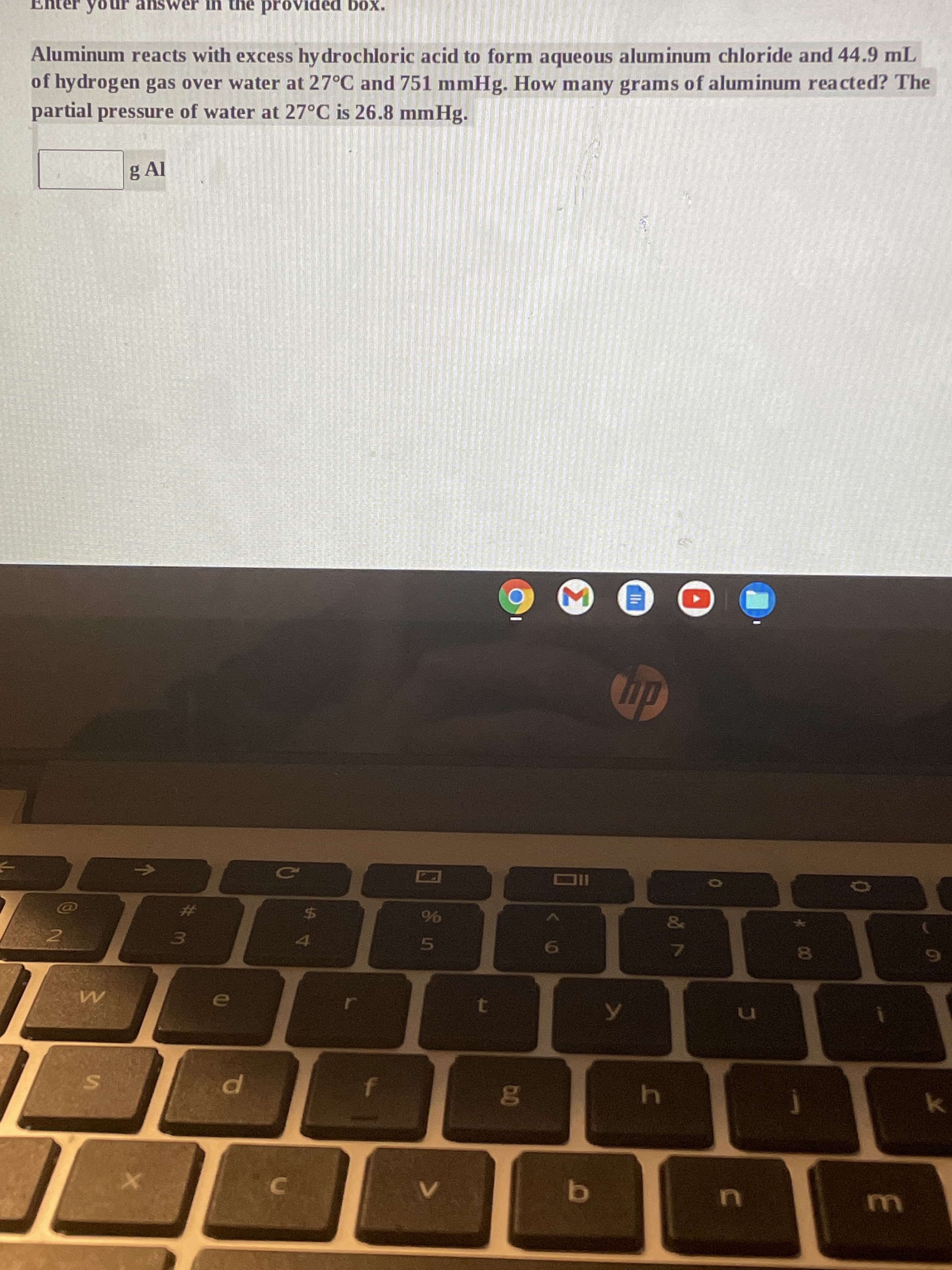
Aluminum reacts with excess hy drochloric acid to form aqueous aluminum chloride and 44.9 mL of hydrogen gas over water at 27°C and 751 mmHg. How many grams of aluminum reacted? The partial pressure of water at 27°C is 26.8 mmHg. g Al
Aluminum reacts with excess hy drochloric acid to form aqueous aluminum chloride and 44.9 mL of hydrogen gas over water at 27°C and 751 mmHg. How many grams of aluminum reacted? The partial pressure of water at 27°C is 26.8 mmHg. g Al
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.93QE: How many square meters will 4.0 L (about 1 gal) of paint cover if it is applied to a uniform...
Related questions
Question
Transcribed Image Text:*00
Pl
nq
4
2.
4.
$4
V<
IIC
g Al
partial pressure of water at 27°C is 26.8 mmHg.
of hydrogen gas over water at 27°C and 751 mmHg. How many grams of aluminum reacted? The
Aluminum reacts with excess hy drochloric acid to form aqueous aluminum chloride and 44.9 mL
answer in
"Xog papiAo.d əyn u JƏ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning