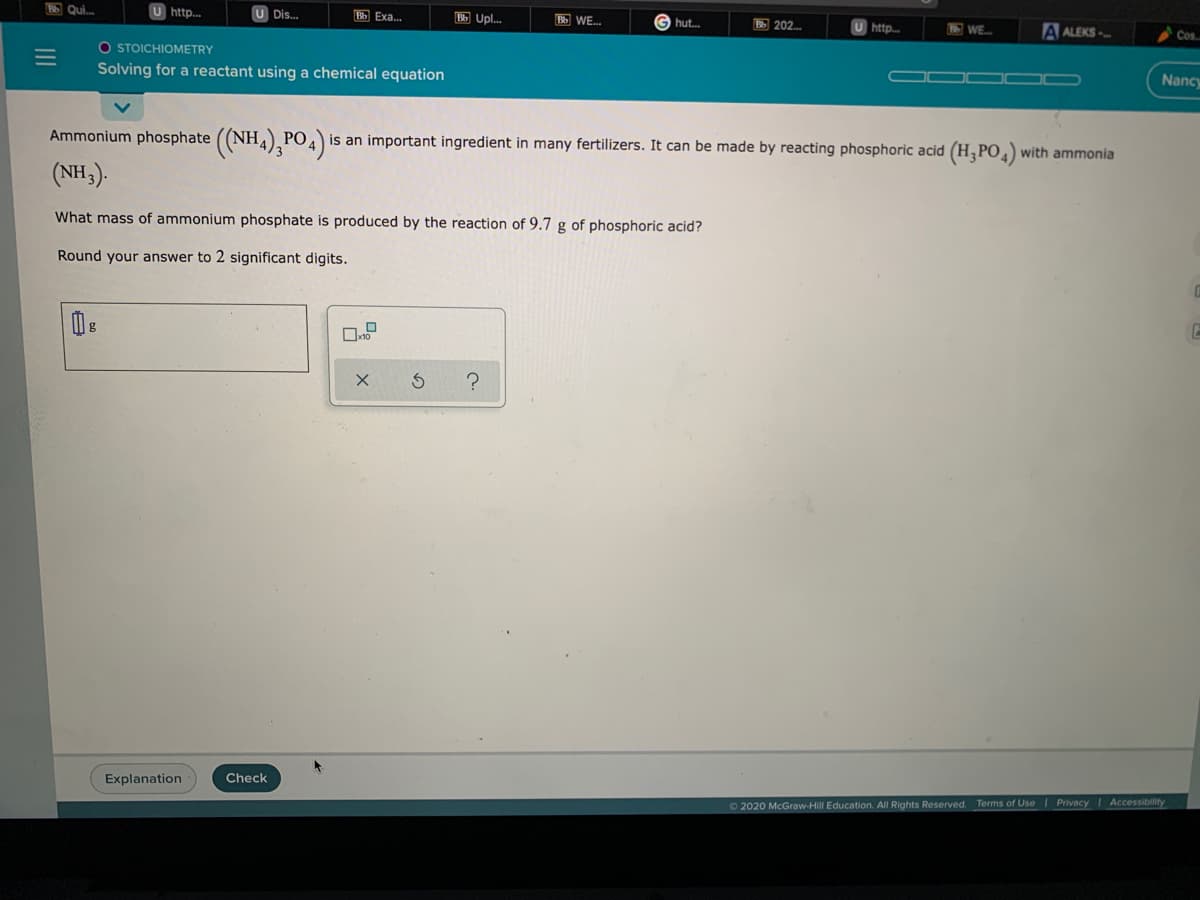
Ammonium phosphate ((NH,) PO, is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia 3 (NH). What mass of ammonium phosphate is produced by the reaction of 9.7 g of phosphoric acid? Round your answer to 2 significant digits.
Ammonium phosphate ((NH,) PO, is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO,) with ammonia 3 (NH). What mass of ammonium phosphate is produced by the reaction of 9.7 g of phosphoric acid? Round your answer to 2 significant digits.
ChapterU4: Toxins: Stoichiometry, Solution Chemistry, And Acids And Bases
Section: Chapter Questions
Problem 20STP
Related questions
Question
100%
Transcribed Image Text:8Qui..
U http..
U Dis.
E Exa.
Bb Upl.
Bb WE.
hut..
202.
http.
E WE.
A ALEKS
Cos.
O STOICHIOMETRY
Solving for a reactant using a chemical equation
OO OC
Nancy
Ammonium phosphate ((NH4),PO4)
is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO) with ammonia
(NH,).
What mass of ammonium phosphate is produced by the reaction of 9.7 g of phosphoric acid?
Round your answer to 2 significant digits.
Explanation
Check
© 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use
Privacy I Accessibility
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning