An aspirin tablet has a mass of 9820.0 mg. Report the mass of the tablet in: grams: • kilograms: kg • micrograms: x 10 μg • centigrams: cg Only input numbers. Your answers must be expressed to the correct number of significant figures. Any values less than one must have a zero in front of the decimal (e.g. 0.01 not .01).
An aspirin tablet has a mass of 9820.0 mg. Report the mass of the tablet in: grams: • kilograms: kg • micrograms: x 10 μg • centigrams: cg Only input numbers. Your answers must be expressed to the correct number of significant figures. Any values less than one must have a zero in front of the decimal (e.g. 0.01 not .01).
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter2: Measurements And Calculations
Section: Chapter Questions
Problem 116AP
Related questions
Question
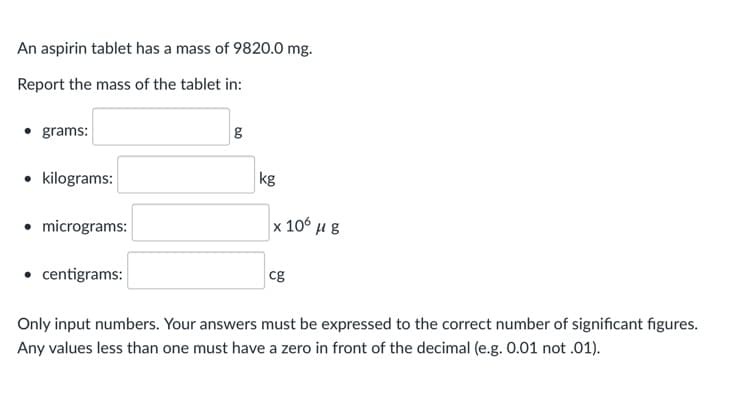
Transcribed Image Text:An aspirin tablet has a mass of 9820.0 mg.
Report the mass of the tablet in:
grams:
• kilograms:
kg
• micrograms:
x 10 μg
• centigrams:
cg
Only input numbers. Your answers must be expressed to the correct number of significant figures.
Any values less than one must have a zero in front of the decimal (e.g. 0.01 not .01).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning