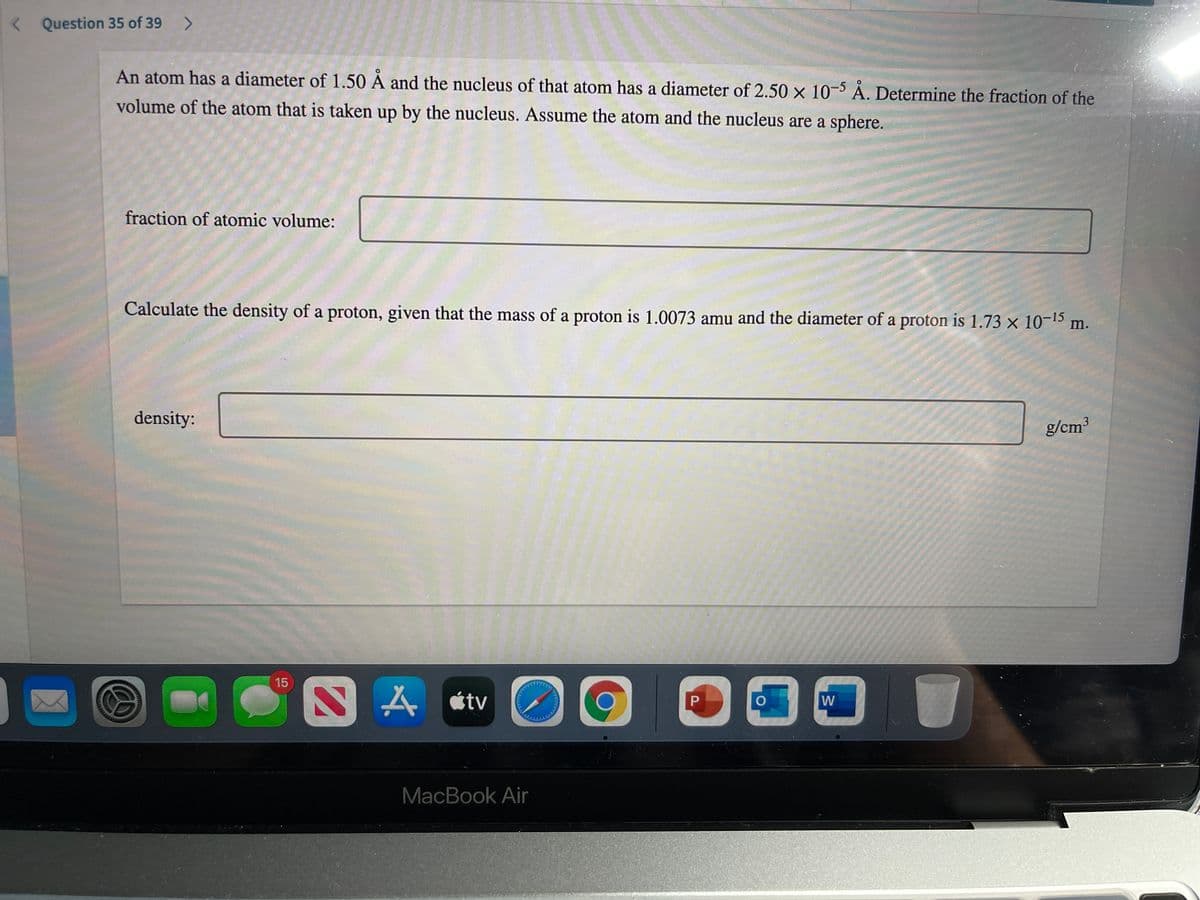
An atom has a diameter of 1.50 À and the nucleus of that atom has a diameter of 2.50 x 10-5 A. Determine the fraction of the volume of the atom that is taken up by the nucleus. Assume the atom and the nucleus are a sphere. fraction of atomic volume: Calculate the density of a proton, given that the mass of a proton is 1.0073 amu and the diameter of a proton is 1.73 x 10-15 m. density: g/cm
An atom has a diameter of 1.50 À and the nucleus of that atom has a diameter of 2.50 x 10-5 A. Determine the fraction of the volume of the atom that is taken up by the nucleus. Assume the atom and the nucleus are a sphere. fraction of atomic volume: Calculate the density of a proton, given that the mass of a proton is 1.0073 amu and the diameter of a proton is 1.73 x 10-15 m. density: g/cm
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter4: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 60QAP: Give three examples of gaseous elements that exist as diatomic molecules. Give three examples of...
Related questions
Question
Transcribed Image Text:< Question 35 of 39
<>
An atom has a diameter of 1.50 À and the nucleus of that atom has a diameter of 2.50 x 10-5 À. Determine the fraction of the
volume of the atom that is taken up by the nucleus. Assume the atom and the nucleus are a sphere.
fraction of atomic volume:
Calculate the density of a proton, given that the mass of a proton is 1.0073 amu and the diameter of a proton is 1.73 x 10-15
m.
density:
g/cm
15
étv
МacBook Air
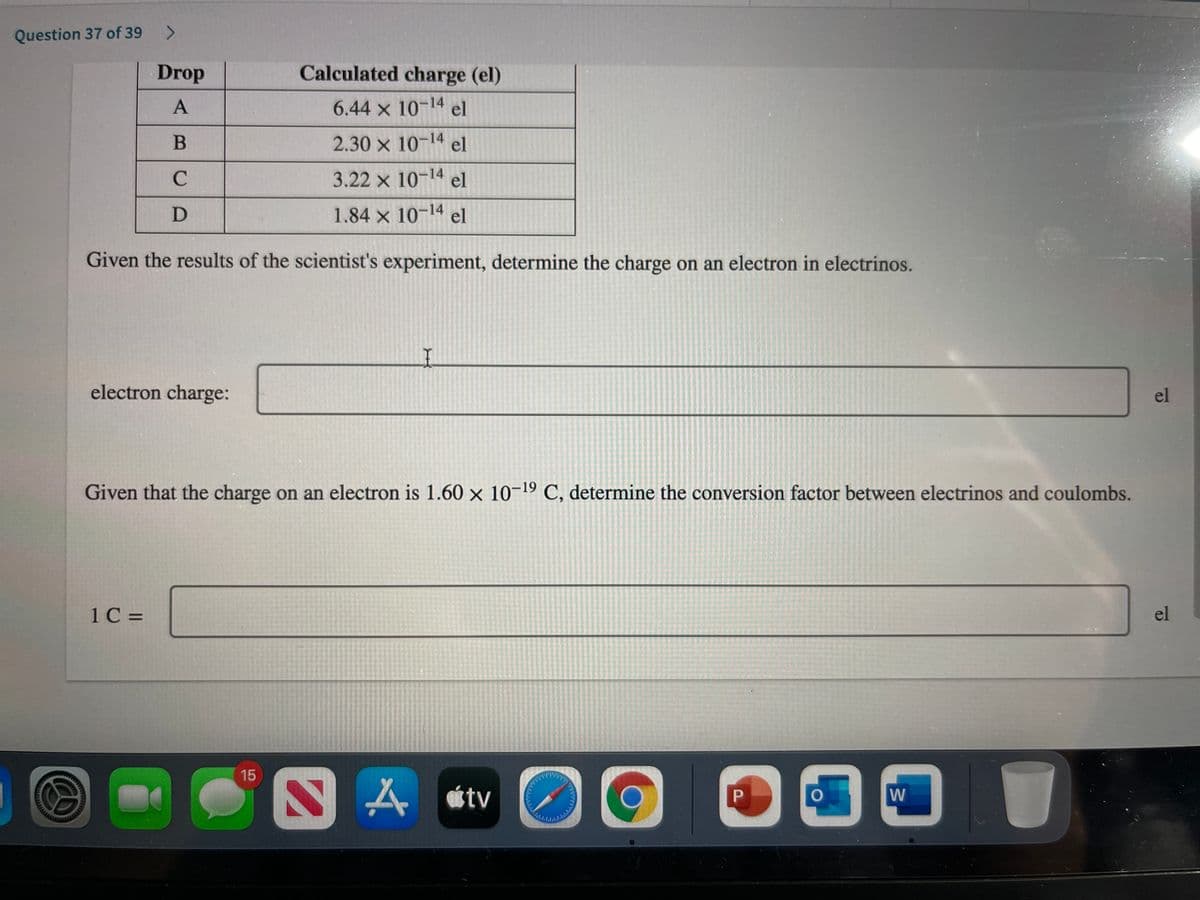
Transcribed Image Text:Question 37 of 39
Drop
Calculated charge (el)
6.44 x 10-14 el
2.30 x 10-14 el
C
3.22 x 10-14 el
1.84 x 10-14 el
Given the results of the scientist's experiment, determine the charge on an electron in electrinos.
electron charge:
el
Given that the charge on an electron is 1.60 x 10-19 C, determine the conversion factor between electrinos and coulombs.
1C =
el
15
A tv
P
w
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning