106. Carbon-12 contains 6 protons and 6 neutrons. The radius of the nucleus is approximately 2.7 fm, and the radius of the atom is approximately 70 pm. Calculate the volume of the nucleus and the volume of the atom. What per- centage of the carbon atom's volume does the nucleus occupy? (Hint: Convert both given radii to m, and then calculate their volumes using the formula for the volume of a sphere, which is V = {rr².)
106. Carbon-12 contains 6 protons and 6 neutrons. The radius of the nucleus is approximately 2.7 fm, and the radius of the atom is approximately 70 pm. Calculate the volume of the nucleus and the volume of the atom. What per- centage of the carbon atom's volume does the nucleus occupy? (Hint: Convert both given radii to m, and then calculate their volumes using the formula for the volume of a sphere, which is V = {rr².)
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter3: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 21A
Related questions
Question
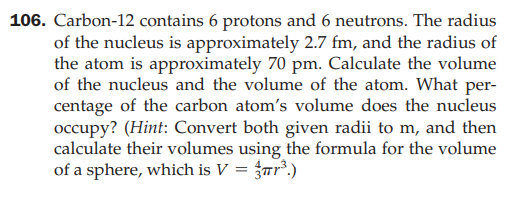
Transcribed Image Text:106. Carbon-12 contains 6 protons and 6 neutrons. The radius
of the nucleus is approximately 2.7 fm, and the radius of
the atom is approximately 70 pm. Calculate the volume
of the nucleus and the volume of the atom. What per-
centage of the carbon atom's volume does the nucleus
occupy? (Hint: Convert both given radii to m, and then
calculate their volumes using the formula for the volume
of a sphere, which is V = {rr².)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax