an be written: СхНу + (x + % у) О2 х СО2 +% у H-О Determine the grams of CO2 output per gram of fuel input for the following alkane fuels: а. Methane — СHA b. Ethane - C2Н6 c. Propane – C3H8
an be written: СхНу + (x + % у) О2 х СО2 +% у H-О Determine the grams of CO2 output per gram of fuel input for the following alkane fuels: а. Methane — СHA b. Ethane - C2Н6 c. Propane – C3H8
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter2: Alkanes And Cycloalkanes
Section: Chapter Questions
Problem 2.57P: Following are heats of combustion per mole for methane, propane, and 2,2,4-trimeth-ylpentane. Each...
Related questions
Question
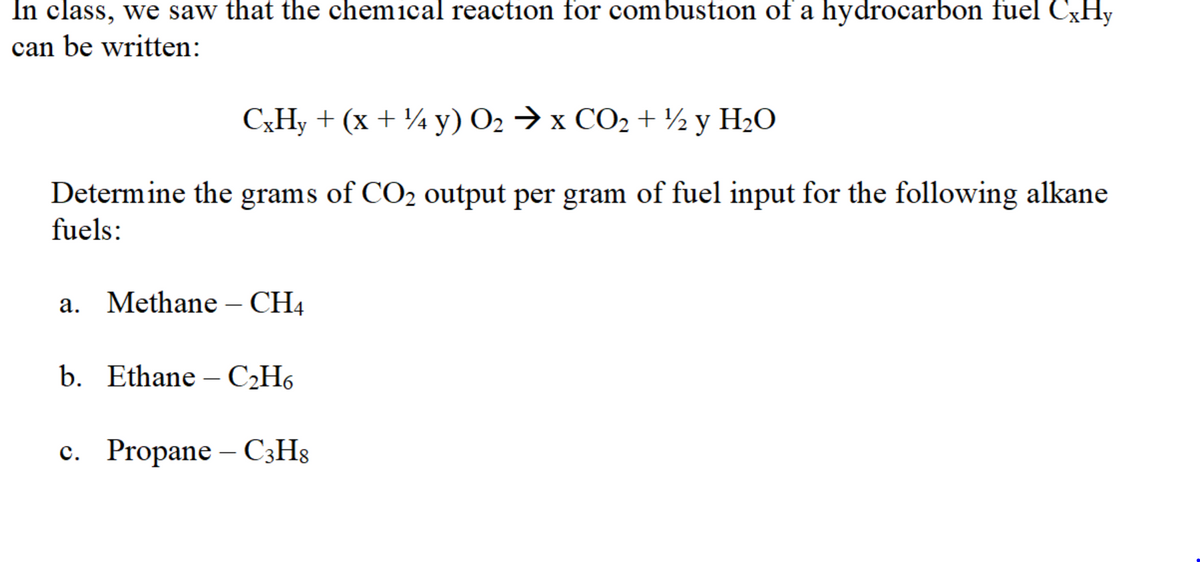
Transcribed Image Text:In class, we saw that the chemical reaction for combustion of a hydrocarbon fuel CXHY
can be written:
C;H, + (x + ¼ y) O2 → x CO2 + ½y H2O
Determine the grams of CO2 output per gram of fuel input for the following alkane
fuels:
a. Methane – CH4
b. Ethane – C2H6
с. Propane
- C3H8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co