The enthalpy for hydrogenation of different isomers of butene is shown in the following figure. Which statement below best interprets this figure? II • H 7k mot 5k mot II + H2 AH-120 kJ mot AH"-115 kJ mot AH-127 kJ mor O GMU 2020 O Stability and hydrogenation enthalpies are not correlated. O Butene Il is less stable than butene I by 7 kJ/mol. O Butene Il 1s 7 kJ/mol more stable thanI and 5 kJ/mol more stable than III. O Butene Il 1s 5 kJ/mol less stable than butene IlI.
The enthalpy for hydrogenation of different isomers of butene is shown in the following figure. Which statement below best interprets this figure? II • H 7k mot 5k mot II + H2 AH-120 kJ mot AH"-115 kJ mot AH-127 kJ mor O GMU 2020 O Stability and hydrogenation enthalpies are not correlated. O Butene Il is less stable than butene I by 7 kJ/mol. O Butene Il 1s 7 kJ/mol more stable thanI and 5 kJ/mol more stable than III. O Butene Il 1s 5 kJ/mol less stable than butene IlI.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 121QRT
Related questions
Question
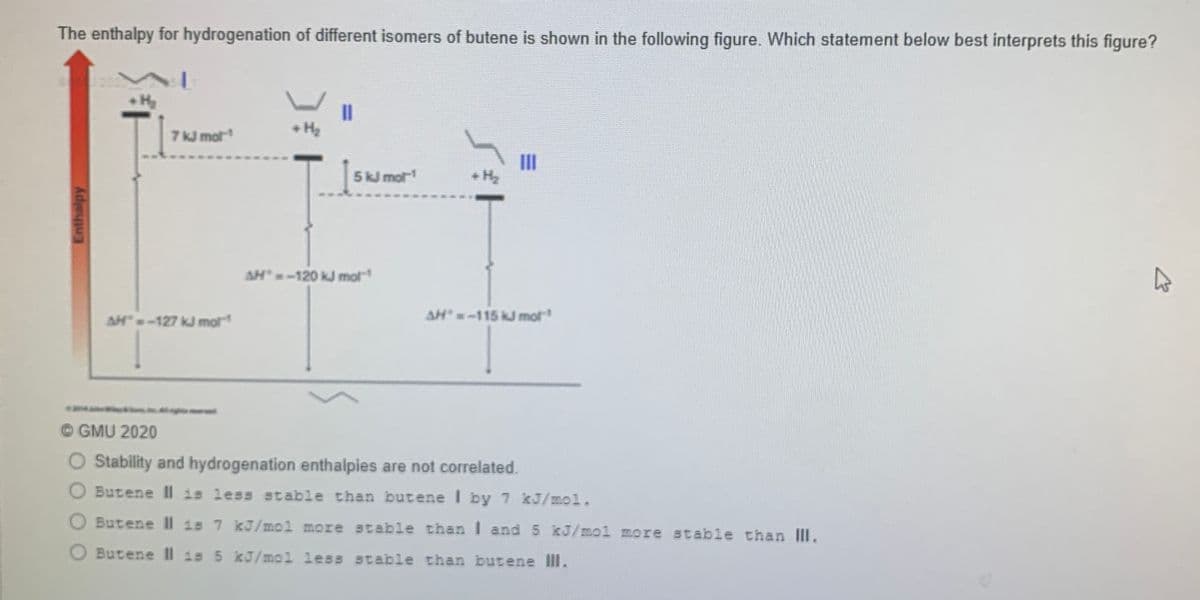
Transcribed Image Text:The enthalpy for hydrogenation of different isomers of butene is shown in the following figure. Which statement below best interprets this figure?
Hy
I3D
H2
7k mot
5kJ mot
+ H2
AH-120 kJ mot
AH"-127 kJ mot
AH-115 kJ mot
© GMU 2020
Stability and hydrogenation enthalpies are not correlated.
Butene II is less stable than butene I by 7 kJ/mol.
O Butene Il is 7 kJ/mol more stable than I and 5 kJ/mol more stable than III.
Butene II is 5 kJ/mol less atable than butene IlI.
Enthalpy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning