an equation relating the rates of change in the 13.29. Write concentrations of the products and reactants in each of the b. following reactions: a. F,(g) + H2O(€) → HOF(g) + HF(2) b. Si(s) + 3 HC1(g) → SIHC1;(t) + H,(e) c. 4 NH3(g) + 3 O2(g) → 2 N2(g) + 6 H,O(g) 13.36. inthe yolatinge rates of change in the
an equation relating the rates of change in the 13.29. Write concentrations of the products and reactants in each of the b. following reactions: a. F,(g) + H2O(€) → HOF(g) + HF(2) b. Si(s) + 3 HC1(g) → SIHC1;(t) + H,(e) c. 4 NH3(g) + 3 O2(g) → 2 N2(g) + 6 H,O(g) 13.36. inthe yolatinge rates of change in the
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section14.1: Rates Of Chemical Reactions
Problem 14.2CYU: What are the relative rates of appearance or disappearance of each product and reactant in the...
Related questions
Question
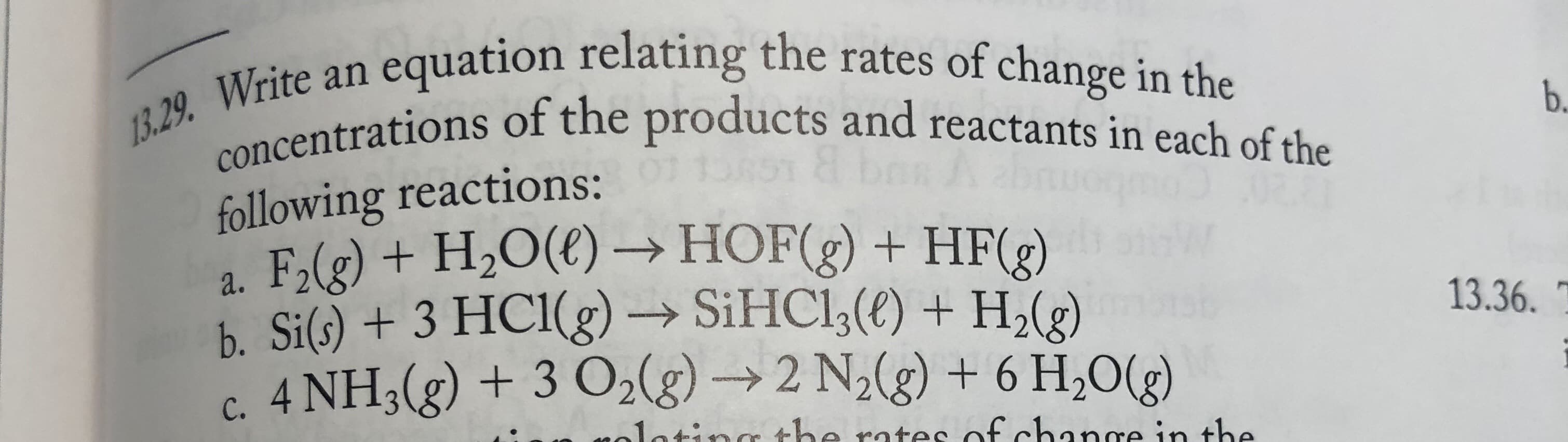
Transcribed Image Text:an equation relating the rates of change in the
13.29. Write
concentrations of the products and reactants in each of the
b.
following reactions:
a. F,(g) + H2O(€) → HOF(g) + HF(2)
b. Si(s) + 3 HC1(g) → SIHC1;(t) + H,(e)
c. 4 NH3(g) + 3 O2(g) → 2 N2(g) + 6 H,O(g)
13.36.
inthe
yolatinge
rates of change in the
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 8 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
