1. A student determines the molar mass of acetone, CH3COH3, by the method used in this experiment. She found that the equilibrium temperature of a mixture of ice and water was 0.5°C on her thermometer. When she added 10.5 g if her sample to the mixture, the temperature, fell to -2.6°C. She then poured off the solution through a screen into a beaker. The mass of the solution was 86.4g. a. What was the freezing point depression? °C b. What was the molality of the acetone? m c. How much acetone was in the decanted solution? d. How much water was in the decanted solution? e. How much acetone would there be in a solution containing 1 kg of water and acetone at the same concentration as she had in her experiment? g acetone f. What did she find to be the molar mass of acetone, assuming she made the calculation properly?
1. A student determines the molar mass of acetone, CH3COH3, by the method used in this experiment. She found that the equilibrium temperature of a mixture of ice and water was 0.5°C on her thermometer. When she added 10.5 g if her sample to the mixture, the temperature, fell to -2.6°C. She then poured off the solution through a screen into a beaker. The mass of the solution was 86.4g. a. What was the freezing point depression? °C b. What was the molality of the acetone? m c. How much acetone was in the decanted solution? d. How much water was in the decanted solution? e. How much acetone would there be in a solution containing 1 kg of water and acetone at the same concentration as she had in her experiment? g acetone f. What did she find to be the molar mass of acetone, assuming she made the calculation properly?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.61QE: When 7.11 g NH4NO3 is added to 100 mL water, the temperature of the calorimeter contents decreases...
Related questions
Question

Transcribed Image Text:1. A student determines the molar mass of acetone, CH3COH3, by the method used in this
experiment. She found that the equilibrium temperature of a mixture of ice and water was
0.5°C on her thermometer. When she added 10.5 g if her sample to the mixture, the
temperature, fell to -2.6°C. She then poured off the solution through a screen into a beaker.
The mass of the solution was 86.4g.
a. What was the freezing point depression?
°C
b. What was the molality of the acetone?
m
c. How much acetone was in the decanted solution?
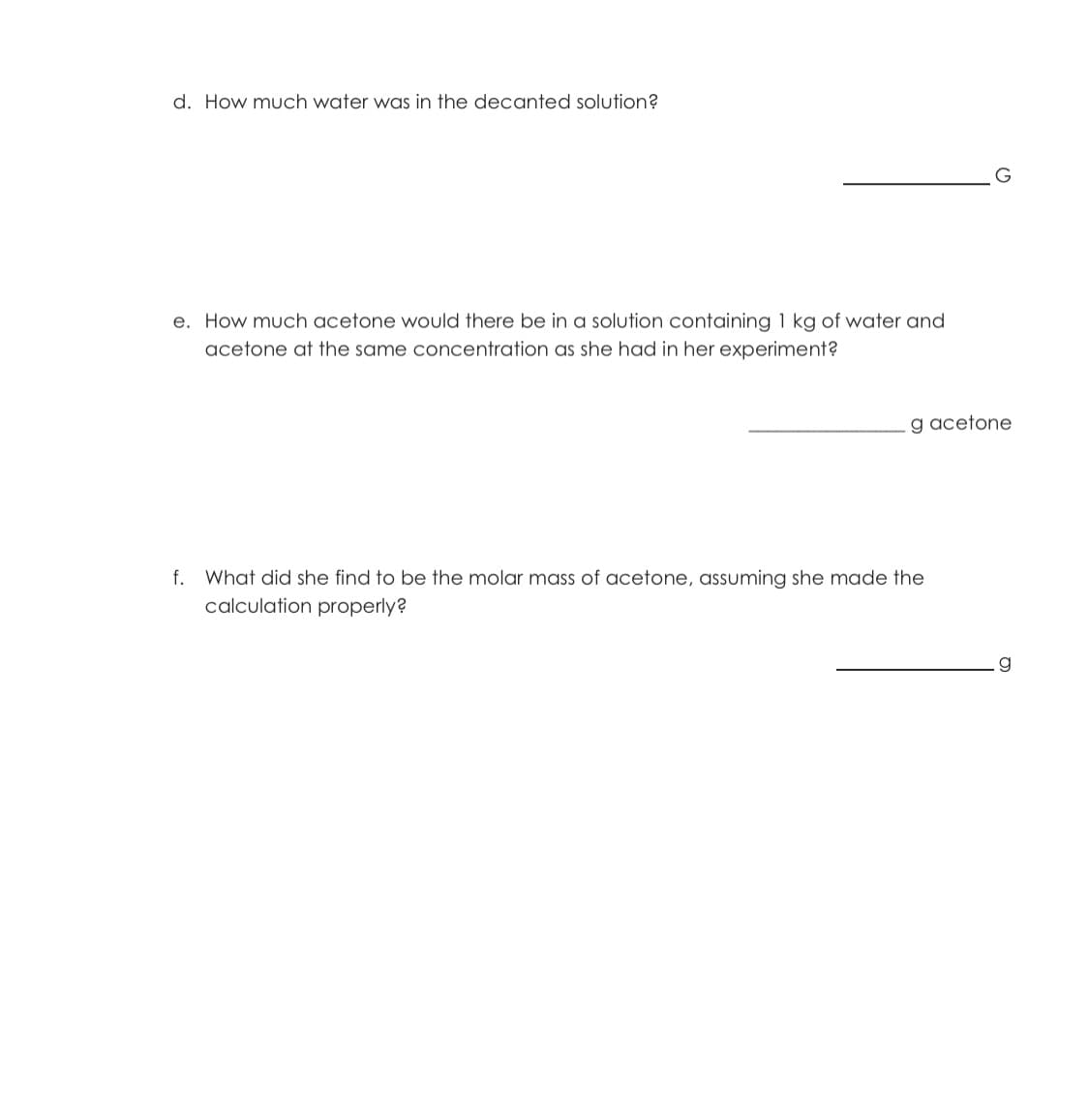
Transcribed Image Text:d. How much water was in the decanted solution?
e. How much acetone would there be in a solution containing 1 kg of water and
acetone at the same concentration as she had in her experiment?
g acetone
f. What did she find to be the molar mass of acetone, assuming she made the
calculation properly?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 8 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning