An ideal diatomic gas has an initial pressure of 1.00×10°P , an initial volume of 2.00m', and an initial temperature of 300K. (This is point 1 on the pV-diagram.) The gas has an isochoric increase in pressure to 2.00x10°Pa. (This is point 2 on the pV-diagram.) The gas then has an isothermal expansion to a volume of 3.00m'. (This is point 3 on the pV-diagram.) The pressure is then reduced adiabatically back down to its original pressure of 1.00×10°P . (This is point 4 on the pV-diagram.) Finally, the gas has an isobaric decrease in volume to its original volume of 2.00m'. (The gas is back to point 1 on the pV-diagram.) a. Fill in the missing values on the following table. Point Volume, Pressure, Temperature, v (m³) p(10ʻPa) T(K) 1 2.00 1.00 300 2 2.00 3 3.00 4 1.00
An ideal diatomic gas has an initial pressure of 1.00×10°P , an initial volume of 2.00m', and an initial temperature of 300K. (This is point 1 on the pV-diagram.) The gas has an isochoric increase in pressure to 2.00x10°Pa. (This is point 2 on the pV-diagram.) The gas then has an isothermal expansion to a volume of 3.00m'. (This is point 3 on the pV-diagram.) The pressure is then reduced adiabatically back down to its original pressure of 1.00×10°P . (This is point 4 on the pV-diagram.) Finally, the gas has an isobaric decrease in volume to its original volume of 2.00m'. (The gas is back to point 1 on the pV-diagram.) a. Fill in the missing values on the following table. Point Volume, Pressure, Temperature, v (m³) p(10ʻPa) T(K) 1 2.00 1.00 300 2 2.00 3 3.00 4 1.00
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter17: Energy In Thermal Processes: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 47P
Related questions
Question
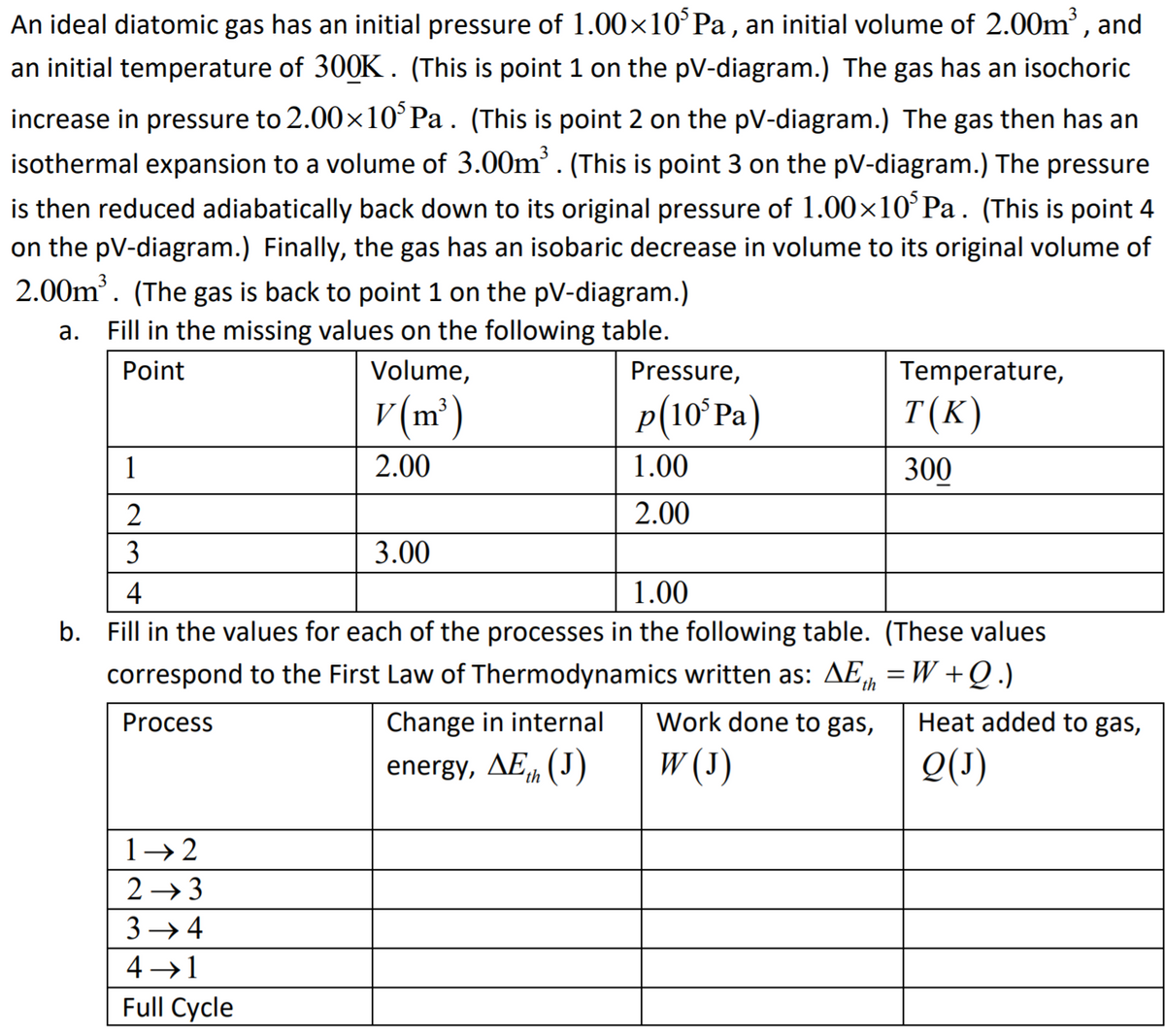
Transcribed Image Text:An ideal diatomic gas has an initial pressure of 1.00×10°Pa , an initial volume of 2.00m', and
an initial temperature of 300K. (This is point 1 on the pV-diagram.) The gas has an isochoric
increase in pressure to 2.00×10°P. . (This is point 2 on the pV-diagram.) The gas then has an
isothermal expansion to a volume of 3.00m'. (This is point 3 on the pV-diagram.) The pressure
is then reduced adiabatically back down to its original pressure of 1.00×10°P.. (This is point 4
on the pV-diagram.) Finally, the gas has an isobaric decrease in volume to its original volume of
2.00m. (The gas is back to point 1 on the pV-diagram.)
а.
Fill in the missing values on the following table.
Point
Volume,
Pressure,
Temperature,
v (m²)
p(10ʻPA)
T(K)
1
2.00
1.00
300
2
2.00
3
3.00
4
1.00
b. Fill in the values for each of the processes in the following table. (These values
correspond to the First Law of Thermodynamics written as: AE, =W +Q.)
Process
Change in internal
Work done to gas,
Heat added to gas,
energy, AE, (J)
W (3)
Q(1)
'th
1→ 2
2 → 3
3 → 4
4 →1
Full Cycle
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning