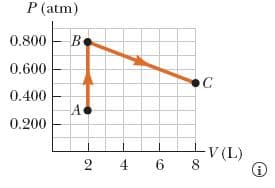
A system consisting of 0.0538 moles of a diatomic ideal gas is taken from state A to state C along the path in the figure below. A pressure-volume graph is plotted on a coordinate plane, where the horizontal axis is V (L), and the vertical axis is P (atm). The path consists of two line segments: a segment from point A (2,0.300) to point B (2,0.800) a segment from point B (2,0.800) to point C (8,0.500) Arrows along the path are aligned such that their tails are closer to point A than are their tips. (a) How much work is done on the gas during this process? J (b) What is the lowest temperature of the gas during this process? K Where does it occur? Point APoint B Point C (c) Find the change in internal energy of the gas in going from A to C. Hint: Adapt the equation (for the change in internal energy of a monatomic ideal gas) ΔU = 3 2nRΔT = 3 2Δ(PV) = 3 2(PCVC − PAVA) to a diatomic ideal gas. J (d) Find the energy delivered to the gas in going from A to C. J
A system consisting of 0.0538 moles of a diatomic ideal gas is taken from state A to state C along the path in the figure below. A pressure-volume graph is plotted on a coordinate plane, where the horizontal axis is V (L), and the vertical axis is P (atm). The path consists of two line segments: a segment from point A (2,0.300) to point B (2,0.800) a segment from point B (2,0.800) to point C (8,0.500) Arrows along the path are aligned such that their tails are closer to point A than are their tips. (a) How much work is done on the gas during this process? J (b) What is the lowest temperature of the gas during this process? K Where does it occur? Point APoint B Point C (c) Find the change in internal energy of the gas in going from A to C. Hint: Adapt the equation (for the change in internal energy of a monatomic ideal gas) ΔU = 3 2nRΔT = 3 2Δ(PV) = 3 2(PCVC − PAVA) to a diatomic ideal gas. J (d) Find the energy delivered to the gas in going from A to C. J
Chapter7: Electricity
Section: Chapter Questions
Problem 14P
Related questions
Question
A system consisting of 0.0538 moles of a diatomic ideal gas is taken from state A to state C along the path in the figure below.
A pressure-volume graph is plotted on a coordinate plane, where the horizontal axis is V (L), and the vertical axis is P (atm). The path consists of two line segments:
- a segment from point A (2,0.300) to point B (2,0.800)
- a segment from point B (2,0.800) to point C (8,0.500)
(a) How much work is done on the gas during this process?
J
(b) What is the lowest temperature of the gas during this process?
K
Where does it occur?
(c) Find the change in internal energy of the gas in going from A to C. Hint: Adapt the equation (for the change in internal energy of a monatomic ideal gas)
J
(d) Find the energy delivered to the gas in going from A to C.
J
J
(b) What is the lowest temperature of the gas during this process?
K
Where does it occur?
Point APoint B Point C
(c) Find the change in internal energy of the gas in going from A to C. Hint: Adapt the equation (for the change in internal energy of a monatomic ideal gas)
ΔU =
nRΔT =
Δ(PV) =
(PCVC − PAVA)
to a diatomic ideal gas.| 3 |
| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
J
(d) Find the energy delivered to the gas in going from A to C.
J
Transcribed Image Text:P (atm)
0.800
Be
0.600
0.400
A
0.200
-V (L)
8
2
4
6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning
