An important reaction that takes place in a blast furnace during the production of iron is the formation of iron metal and CO2 from Fe2O3 and СО. Part A Find the mass of Fe2 O3 required to form 950 kg of iron. Express your answer with the appropriate units. HA MFE2O3 = Value Units
An important reaction that takes place in a blast furnace during the production of iron is the formation of iron metal and CO2 from Fe2O3 and СО. Part A Find the mass of Fe2 O3 required to form 950 kg of iron. Express your answer with the appropriate units. HA MFE2O3 = Value Units
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter9: Gases
Section: Chapter Questions
Problem 86E: During the discussion of gaseous diffusion for enriching uranium, it was claimed that 235UF6...
Related questions
Question
Please answer question 20 part A and B
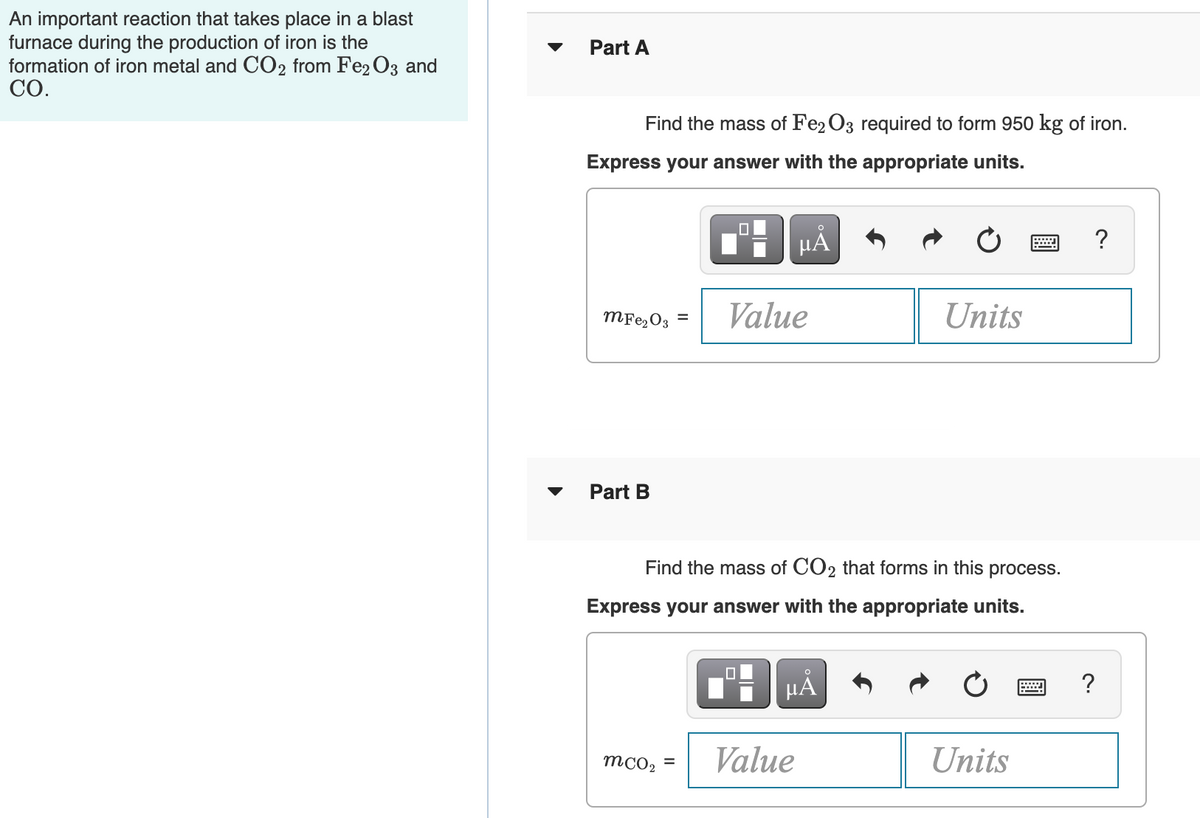
Transcribed Image Text:An important reaction that takes place in a blast
furnace during the production of iron is the
formation of iron metal and CO2 from Fe2 O3 and
СО.
Part A
Find the mass of Fe2 O3 required to form 950 kg of iron.
Express your answer with the appropriate units.
HẢ
?
MF22O3 =
Value
Units
Part B
Find the mass of CO2 that forms in this process.
Express your answer with the appropriate units.
?
mcO2 =
Value
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning