An insulated Thermos contains 200 cm³ of hot coffee at 81.0°C. You put in a 14.0 g ice cube at its melting point to cool the coffee. By how many degrees has your coffee cooled once the ice has melted and equilibrium is reached? Treat the coffee as though it were pure water and neglect energy exchanges with the environment. The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. The density of water is 1.00 g/cm³. Number i Units
An insulated Thermos contains 200 cm³ of hot coffee at 81.0°C. You put in a 14.0 g ice cube at its melting point to cool the coffee. By how many degrees has your coffee cooled once the ice has melted and equilibrium is reached? Treat the coffee as though it were pure water and neglect energy exchanges with the environment. The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333 kJ/kg. The density of water is 1.00 g/cm³. Number i Units
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 100P: One easy way to reduce heating (and cooling) costs is to add extra insulation in the attic of a...
Related questions
Question
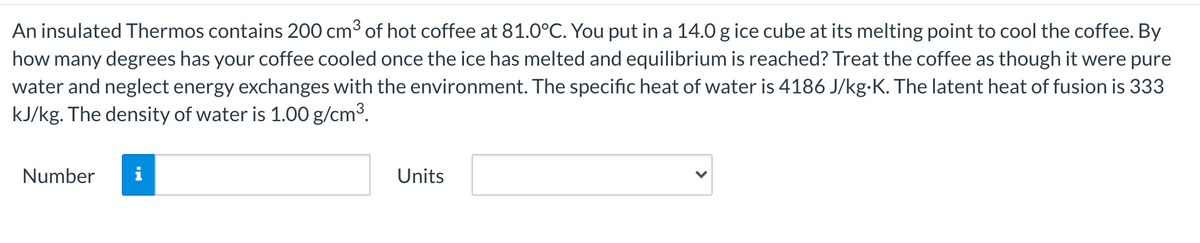
Transcribed Image Text:An insulated Thermos contains 200 cm3 of hot coffee at 81.0°C. You put in a 14.0 g ice cube at its melting point to cool the coffee. By
how many degrees has your coffee cooled once the ice has melted and equilibrium is reached? Treat the coffee as though it were pure
water and neglect energy exchanges with the environment. The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333
kJ/kg. The density of water is 1.00 g/cm3.
Number
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning