An organic compound weighing 0.6827 g is decomposed, giving 0.2095 g carbon and 0.08436 g hydrogen. What are the percentages of hydrogen and carbon in this compound? = H% i %C = i It is likely that the compound contains another element since the percentages do not add up to 100% eTextbook and Media Hint Save for L ater Attempts: 2 of : used Submit Answer
An organic compound weighing 0.6827 g is decomposed, giving 0.2095 g carbon and 0.08436 g hydrogen. What are the percentages of hydrogen and carbon in this compound? = H% i %C = i It is likely that the compound contains another element since the percentages do not add up to 100% eTextbook and Media Hint Save for L ater Attempts: 2 of : used Submit Answer
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 120AP
Related questions
Question
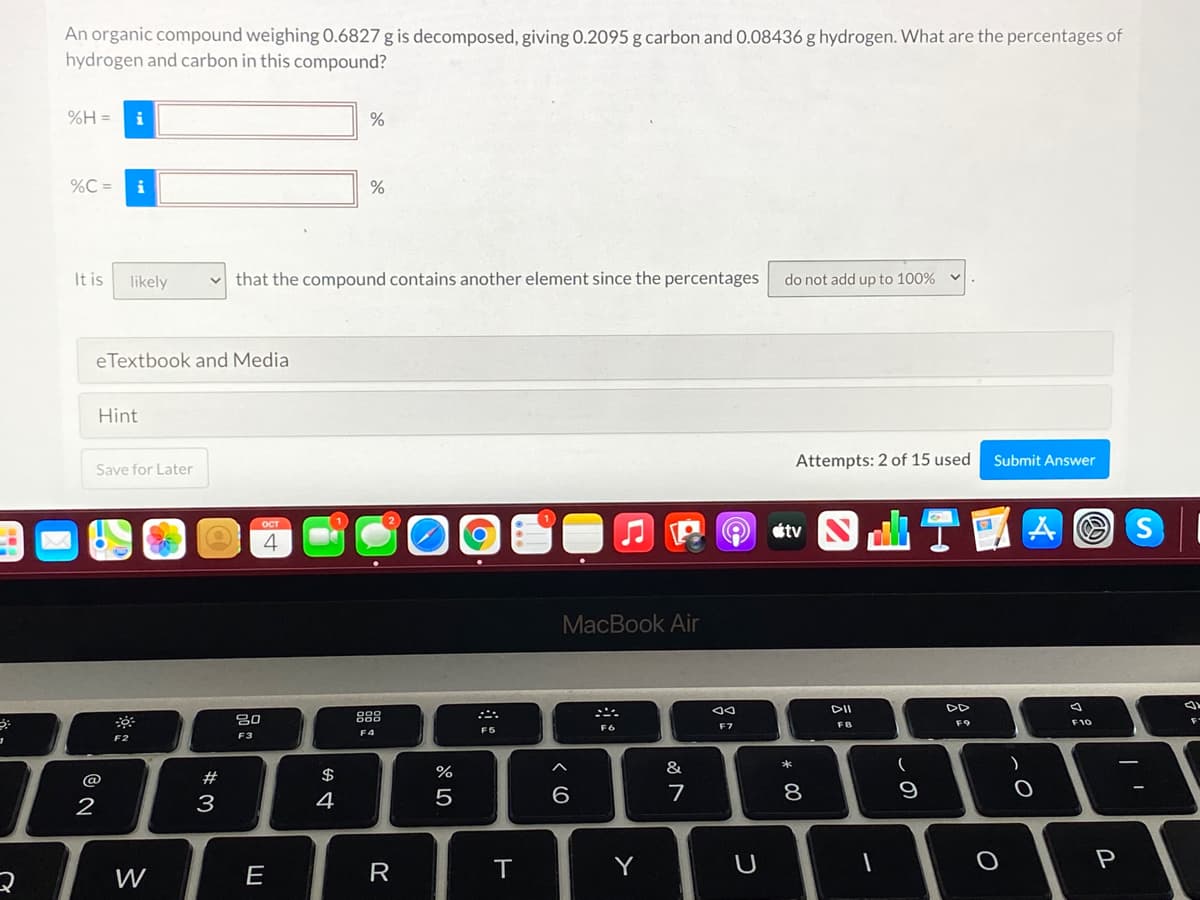
Transcribed Image Text:An organic compound weighing 0.6827 g is decomposed, giving 0.2095 g carbon and 0.08436 g hydrogen. What are the percentages of
hydrogen and carbon in this compound?
%H =
i
%
%C =
i
It is
likely
that the compound contains another element since the percentages
do not add up to 100%
eTextbook and Media
Hint
Attempts: 2 of 15 used
Submit Answer
Save for Later
A O S
étv
MacBook Air
DD
80
888
F9
F10
F7
F5
F2
F3
&
@
#
$
7
8
9
2
3
4
R
T
Y
P
W
E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning