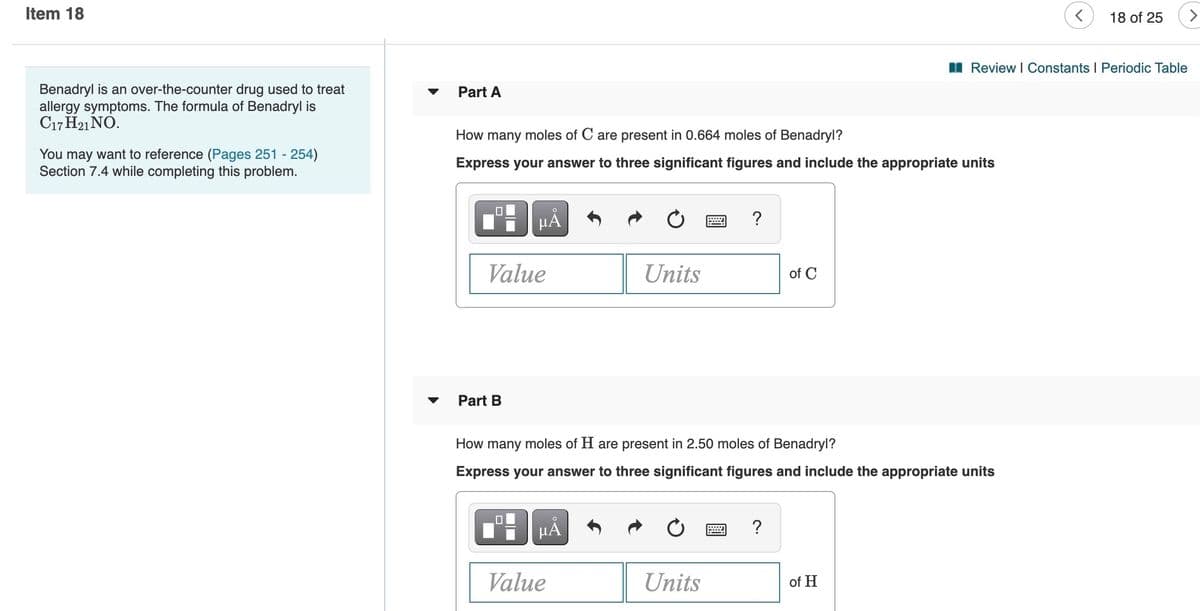
I Review I Constants I Periodic Ta Benadryl is an over-the-counter drug used to treat allergy symptoms. The formula of Benadryl is Cı7H21NO. Part A How many moles of C are present in 0.664 moles of Benadryl? You may want to reference (Pages 251 - 254) Section 7.4 while completing this problem. Express your answer to three significant figures and include the appropriate units HA ? Value Units of C Part B How many moles of H are present in 2.50 moles of Benadryl? Express your answer to three significant figures and include the appropriate units HA Value Units of H
I Review I Constants I Periodic Ta Benadryl is an over-the-counter drug used to treat allergy symptoms. The formula of Benadryl is Cı7H21NO. Part A How many moles of C are present in 0.664 moles of Benadryl? You may want to reference (Pages 251 - 254) Section 7.4 while completing this problem. Express your answer to three significant figures and include the appropriate units HA ? Value Units of C Part B How many moles of H are present in 2.50 moles of Benadryl? Express your answer to three significant figures and include the appropriate units HA Value Units of H
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter33: Preparation Of Copper(i) Chloride
Section: Chapter Questions
Problem 5ASA
Related questions
Question
Transcribed Image Text:Item 18
18 of 25
>
I Review I Constants I Periodic Table
Benadryl is an over-the-counter drug used to treat
allergy symptoms. The formula of Benadryl is
C17 H21 NÓ.
Part A
How many moles of C are present in 0.664 moles of Benadryl?
You may want to reference (Pages 251 - 254)
Section 7.4 while completing this problem.
Express your answer to three significant figures and include the appropriate units
HẢ
Value
Units
of C
Part B
How many moles of H are present in 2.50 moles of Benadryl?
Express your answer to three significant figures and include the appropriate units
HẢ
?
Value
Units
of H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax