How many moles of H2 can be produced from z grams of Mg in magnesium-aluminum alloy? The molar mass of Mg is 24.31 g mol1. Express your answer in terms of z to four decimal places (i.e., 0.5000x). • View Available Hint(s) ? mol H2 Submit Part B How many moles of H2 can be produced from y grams of Al in magnesium-aluminum alloy? The molar mass of Al is 26.98 g mol-1. Express your answer in terms of y to four decimal places (i.e., 0.5000y). • View Available Hint(s)
How many moles of H2 can be produced from z grams of Mg in magnesium-aluminum alloy? The molar mass of Mg is 24.31 g mol1. Express your answer in terms of z to four decimal places (i.e., 0.5000x). • View Available Hint(s) ? mol H2 Submit Part B How many moles of H2 can be produced from y grams of Al in magnesium-aluminum alloy? The molar mass of Al is 26.98 g mol-1. Express your answer in terms of y to four decimal places (i.e., 0.5000y). • View Available Hint(s)
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter11: Stoichiometry
Section: Chapter Questions
Problem 64A
Related questions
Question
please answer this properly and use the units that are asked in the answer please don't use anything else ASAp 5ab please
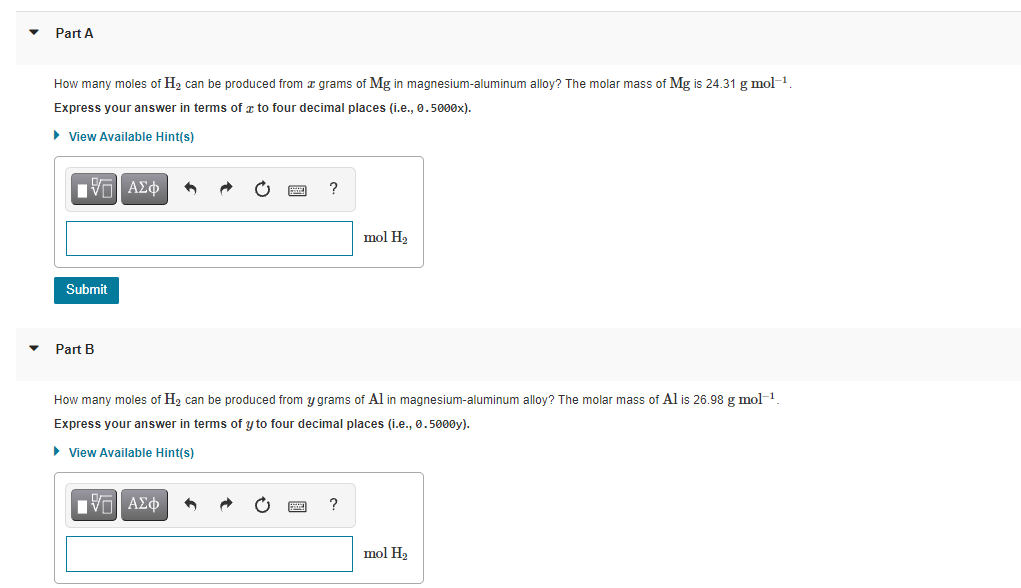
Transcribed Image Text:Part A
How many moles of H2 can be produced from z grams of Mg in magnesium-aluminum alloy? The molar mass of Mg is 24.31 g mol-1.
Express your answer in terms of z to four decimal places (i.e., 0.5000x).
• View Available Hint(s)
Πν ΑΣφ
?
mol H2
Submit
Part B
How many moles of H2 can be produced from y grams of Al in magnesium-aluminum alloy? The molar mass of Al is 26.98 g mol-1
Express your answer in terms of y to four decimal places (i.e., 0.5000y).
• View Available Hint(s)
Πνα ΑΣφ
mol H2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole