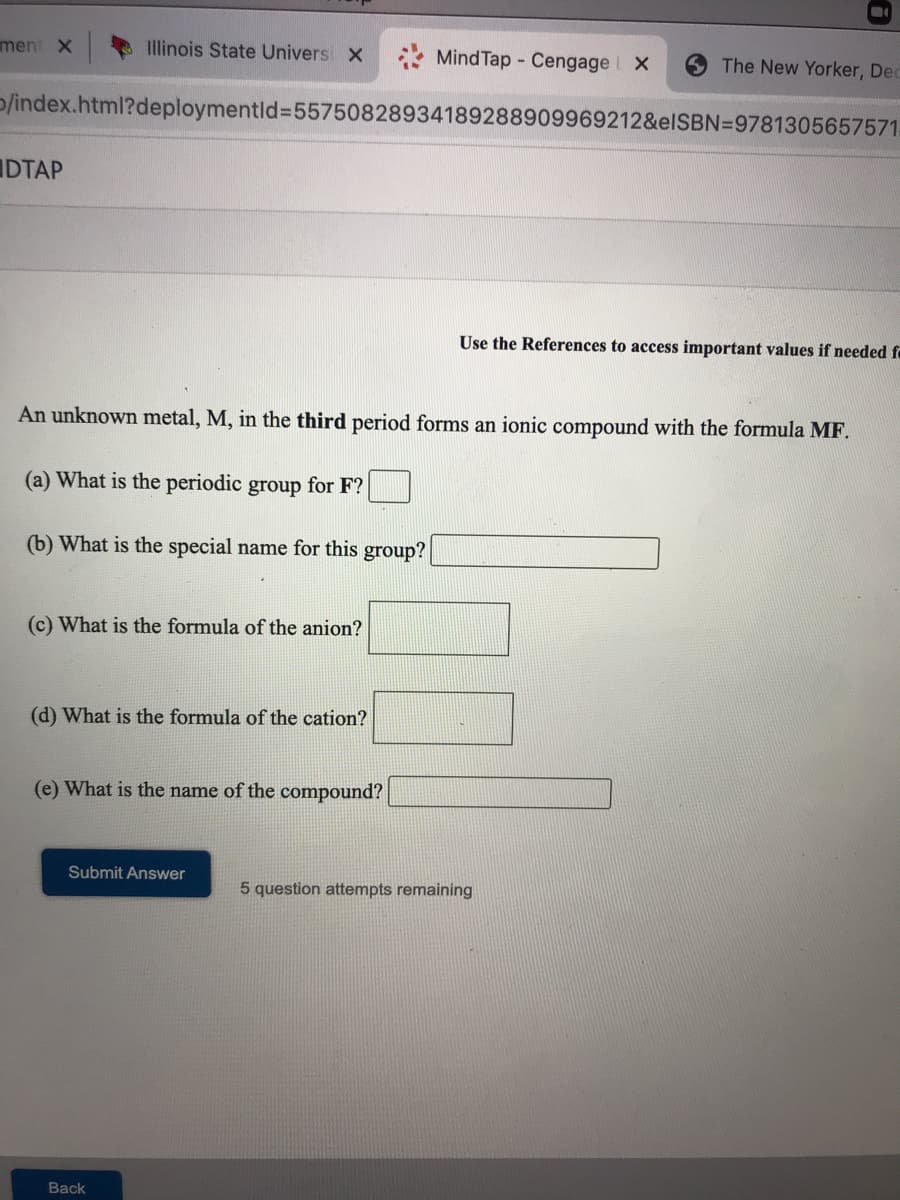
An unknown metal, M, in the third period forms an ionic compound with the formula MF. (a) What is the periodic group for F? (b) What is the special name for this group? (c) What is the formula of the anion?
An unknown metal, M, in the third period forms an ionic compound with the formula MF. (a) What is the periodic group for F? (b) What is the special name for this group? (c) What is the formula of the anion?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 111CP
Related questions
Question
Transcribed Image Text:Illinois State Univers X
Mind Tap Cengage
ment X
The New Yorker, Dec
p/index.html?deploymentld%3D55750828934189288909969212&elSBN=9781305657571.
IDTAP
Use the References to access important values if needed f
An unknown metal, M, in the third period forms an ionic compound with the formula MF.
(a) What is the periodic group for F?
(b) What is the special name for this group?
(c) What is the formula of the anion?
(d) What is the formula of the cation?
(e) What is the name of the compound?
Submit Answer
5 question attempts remaining
Back
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax