Which of the following statements about atomic structure is true? O The periodic table arranges elements by increasing atomic mass The Bohr-Rutherford model shows electrons orbiting the nucleus. The atomic number of an element is the sum of the number of protons and neutrons. The mass number of an element is the number of protons in the nucleus. If magnesium is added to a solution of silver nitrate, which of the following would you expect to occur? The reaction will proceed slowly and result in the formation of magnesium nitrate and silver. The reaction will not proceed. The reaction will proceed quickly and result in the formation of magnesium nitrate and silver. none of the above
Which of the following statements about atomic structure is true? O The periodic table arranges elements by increasing atomic mass The Bohr-Rutherford model shows electrons orbiting the nucleus. The atomic number of an element is the sum of the number of protons and neutrons. The mass number of an element is the number of protons in the nucleus. If magnesium is added to a solution of silver nitrate, which of the following would you expect to occur? The reaction will proceed slowly and result in the formation of magnesium nitrate and silver. The reaction will not proceed. The reaction will proceed quickly and result in the formation of magnesium nitrate and silver. none of the above
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 73QAP: Criticize each of the following statements: (a) The Rutherford experiment confirmed the presence of...
Related questions
Question
100%
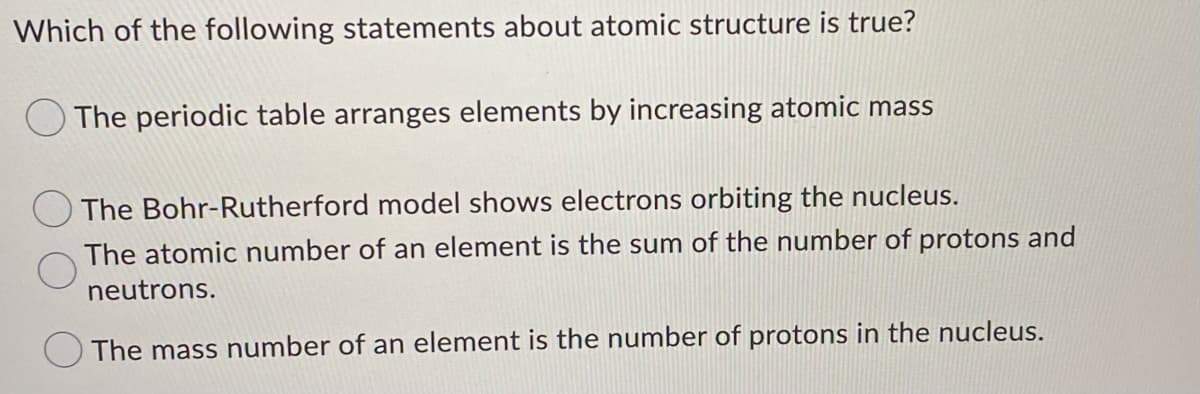
Transcribed Image Text:Which of the following statements about atomic structure is true?
O The periodic table arranges elements by increasing atomic mass
The Bohr-Rutherford model shows electrons orbiting the nucleus.
The atomic number of an element is the sum of the number of protons and
neutrons.
The mass number of an element is the number of protons in the nucleus.

Transcribed Image Text:If magnesium is added to a solution of silver nitrate, which of the following would
you expect to occur?
The reaction will proceed slowly and result in the formation of magnesium
nitrate and silver.
The reaction will not proceed.
The reaction will proceed quickly and result in the formation of magnesium
nitrate and silver.
none of the above
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning
