An unknown reaction was observed, and the following data were collected: T k (К) (M 1.s 1) 352 109 426 185 Determine the activation energy for this reaction.
An unknown reaction was observed, and the following data were collected: T k (К) (M 1.s 1) 352 109 426 185 Determine the activation energy for this reaction.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 98CWP: A certain reaction has the form aAProducts At a particular temperature, concentration versus time...
Related questions
Question
Transcribed Image Text:Review Constants Periodic Tal
III K
III A
9.
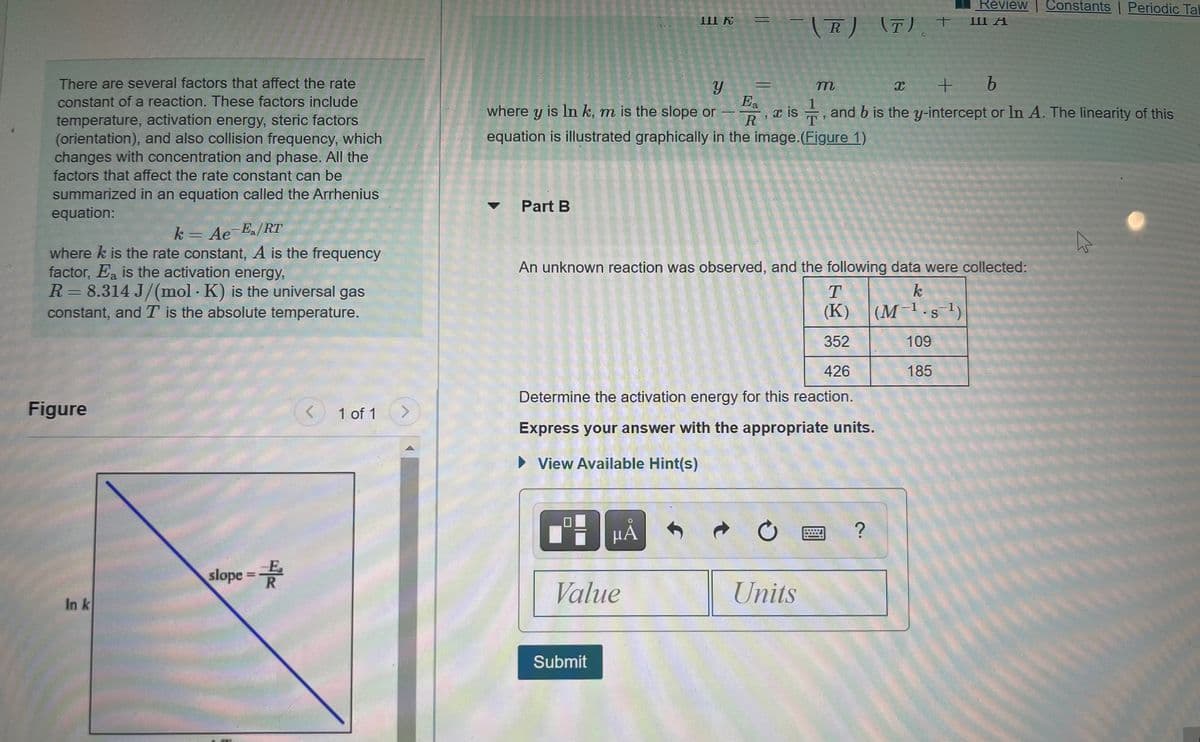
There are several factors that affect the rate
constant of a reaction. These factors include
т
Ea
where y is In k, m is the slope or
x is
-, and b is the y-intercept or In A. The linearity of this
temperature, activation energy, steric factors
(orientation), and also collision frequency, which
changes with concentration and phase. All the
factors that affect the rate constant can be
R
T
equation is illustrated graphically in the image.(Figure 1)
summarized in an equation called the Arrhenius
equation:
Part B
k = Ae Ea/RT
where k is the rate constant, A is the frequency
factor, Ea is the activation energy,
R= 8.314 J/(mol · K) is the universal gas
constant, and T is the absolute temperature.
An unknown reaction was observed, and the following data were collected:
k.
(K)
(M-1.s 1)
352
109
426
185
Determine the activation energy for this reaction.
Figure
1 of 1
Express your answer with the appropriate units.
View Available Hint(s)
slope =
Value
Units
In k
Submit
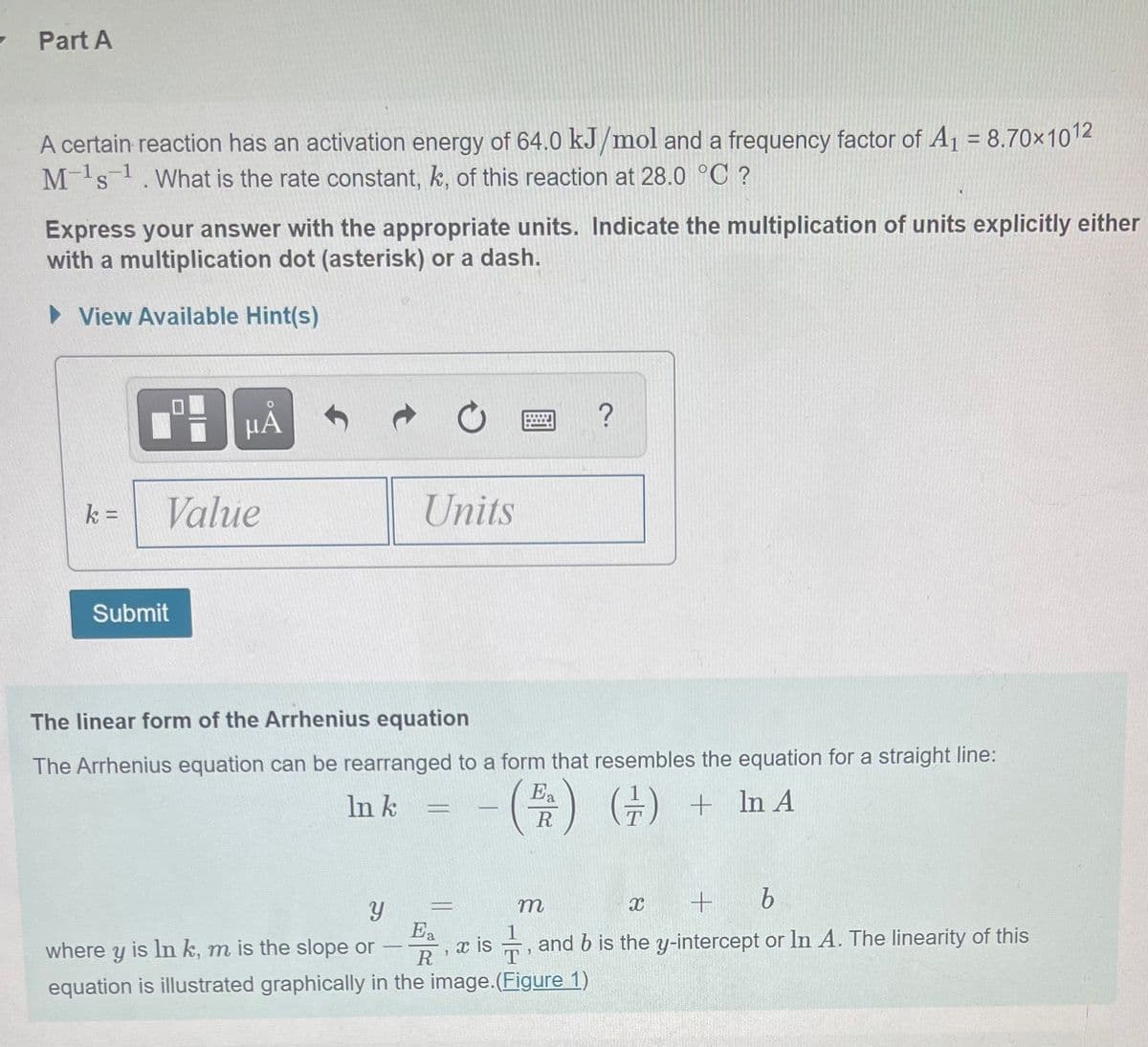
Transcribed Image Text:Part A
A certain reaction has an activation energy of 64.0 kJ/mol and a frequency factor of A1 = 8.70x1012
Ms.What is the rate constant, k, of this reaction at 28.0 °C ?
%3D
Express your answer with the appropriate units. Indicate the multiplication of units explicitly either
with a multiplication dot (asterisk) or a dash.
> View Available Hint(s)
HẢ
k =
Value
Units
Submit
The linear form of the Arrhenius equation
The Arrhenius equation can be rearranged to a form that resembles the equation for a straight line:
Ea
In k
(금) + InA
In A
R
m
Ea
where y is In k, m is the slope or
x is , and b is the y-intercept or In A. The linearity of this
R
T
equation is illustrated graphically in the image.(Figure 1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning