Analysis 5 ppm Fe (III) Is done with a hew analysis method. The experiment is repeated 3 times, and the average iron ion concentration is determined as 4.7 ppm. The standard deviation, s = 0.1, is calculated according to the results of the three experiments. Does the new analysis method contain systematic errors at a 95% confidence level? (Choose the t value from the Table) t values Degrees of freedom 95% CL 12.7 4.3 3.18 2.78 O a. A systematic error exists O b. Only random error exists
Analysis 5 ppm Fe (III) Is done with a hew analysis method. The experiment is repeated 3 times, and the average iron ion concentration is determined as 4.7 ppm. The standard deviation, s = 0.1, is calculated according to the results of the three experiments. Does the new analysis method contain systematic errors at a 95% confidence level? (Choose the t value from the Table) t values Degrees of freedom 95% CL 12.7 4.3 3.18 2.78 O a. A systematic error exists O b. Only random error exists
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.18QAP
Related questions
Question
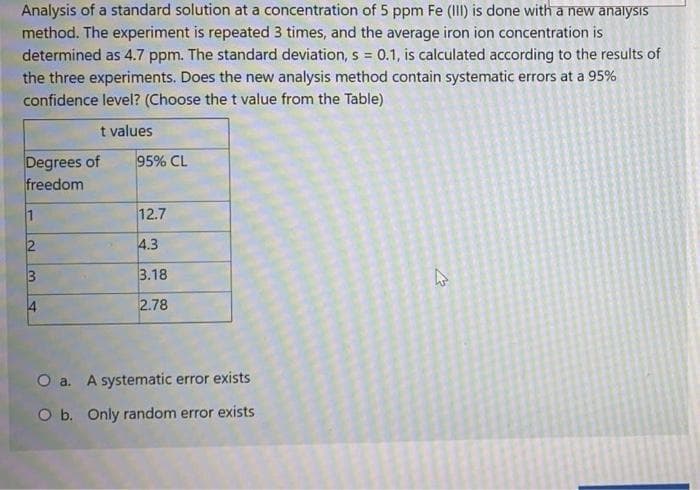
Transcribed Image Text:Analysis of a standard solution at a concentration of 5 ppm Fe (III) is done with a new analysis
method. The experiment is repeated 3 times, and the average iron ion concentration is
determined as 4.7 ppm. The standard deviation, s = 0.1, is calculated according to the results of
the three experiments. Does the new analysis method contain systematic errors at a 95%
confidence level? (Choose the t value from the Table)
t values
Degrees of
freedom
95% CL
1
12.7
4.3
3.18
4
2.78
O a. A systematic error exists
O b. Only random error exists
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you







Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning