D. 10 mL volumetric flask Mass of volumetric flask Volume of water measured in each trial 10:00mL Calculated density of water (g/mL) Mass of volumetric Trial flask and water (g) Mass of water (g) 1.1452g/mL 1.1432 9/mL 1 21.452 g 21.4329 21-423g 11.4529 .432 9 3 4 1.116 glmL 21.416 9 Average density - |431 glmL Standard deviation in density Percent error in density
D. 10 mL volumetric flask Mass of volumetric flask Volume of water measured in each trial 10:00mL Calculated density of water (g/mL) Mass of volumetric Trial flask and water (g) Mass of water (g) 1.1452g/mL 1.1432 9/mL 1 21.452 g 21.4329 21-423g 11.4529 .432 9 3 4 1.116 glmL 21.416 9 Average density - |431 glmL Standard deviation in density Percent error in density
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 7E: 7.The word pour is commonly used in reference to liquids but not to solids or gases. Can you pour a...
Related questions
Question
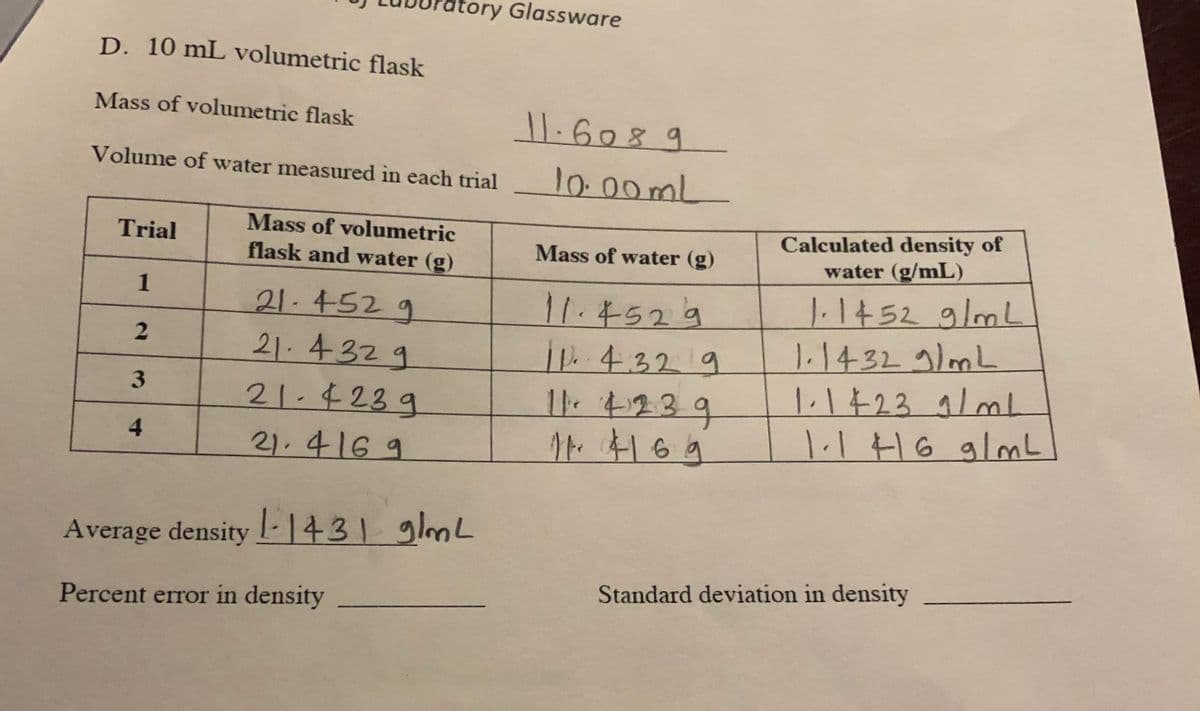
Transcribed Image Text:atory Glassware
D. 10 mL volumetric flask
Mass of volumetric flask
1.6089
Volume of water measured in each trial
10:00 ml
Calculated density of
water (g/mL)
Mass of volumetric
Trial
Mass of water (g)
flask and water (g)
11.5529
ip.432/9
11.4239
1.14529/mL
1.14329/mL
1.1723 9/mL
1.1H6 glmL
21.452 9
21. 4329
21.4239
4.
21.4169
Average density -1431 glmL
Standard deviation in density
Percent error in density
1.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
