You have a stock solution certified by a manufacturer to contain 220.0 + 0.4 ug So /ml. You would like to dilute it by a factor of 100 to obtain 2.200 g/ml. Two possible methods of dilution are stated. For each method, calculate the resulting absolute uncertainty in concentration. Use the manufacturer's tolerances in the table for uncertainties. Glassware Tolerance 1-ml. transfer pipet 20.006 ml. 10-ml transfer pipet 10.02 ml. 100-ml volumetric flask 10.08 ml Method A: Dilute 10.00 ml up to 100 mL with a transfer pipet and volumetric flask. Then take 10.00 mL of the dilute solution and dilute it again to 100 ml. absolute uncertainty: = 14 He/ml. Incemet Method B: Dilute 1.000 mL up 10 100 mL with a transfer pipet and volumetric flask. absolute uncertainty: 0.6 Helml.
You have a stock solution certified by a manufacturer to contain 220.0 + 0.4 ug So /ml. You would like to dilute it by a factor of 100 to obtain 2.200 g/ml. Two possible methods of dilution are stated. For each method, calculate the resulting absolute uncertainty in concentration. Use the manufacturer's tolerances in the table for uncertainties. Glassware Tolerance 1-ml. transfer pipet 20.006 ml. 10-ml transfer pipet 10.02 ml. 100-ml volumetric flask 10.08 ml Method A: Dilute 10.00 ml up to 100 mL with a transfer pipet and volumetric flask. Then take 10.00 mL of the dilute solution and dilute it again to 100 ml. absolute uncertainty: = 14 He/ml. Incemet Method B: Dilute 1.000 mL up 10 100 mL with a transfer pipet and volumetric flask. absolute uncertainty: 0.6 Helml.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.13QAP
Related questions
Question
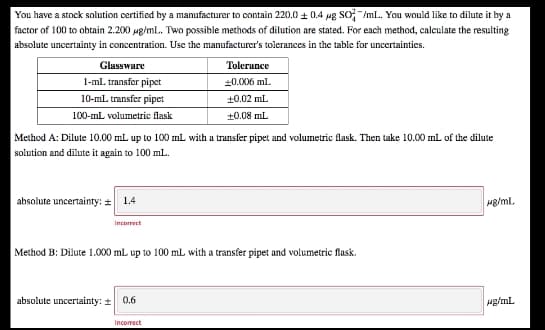
Transcribed Image Text:You have a stock solution certified by a manufacturer to contain 220.0 + 0.4 ug So /mL. You would like to dilute it by a
factor of 100 to obtain 2.200 ug/mL.. Two possible methods of dilution are stated. For each method, calculate the resulting
absolute uncertainty in concentration. Use the manufacturer's tolerances in the table for uncertainties.
Glassware
Tolerance
1-ml. transfer pipet
+0.006 ml.
10-ml transfer pipet
10.02 mL
100-ml volumetric flask
+0.08 mL
Method A: Dilute 10.00 mL up to 100 mL with a transfer pipet and volumetric flask. Then take 10.00 ml of the dilute
solution and dilute it again to 100 mL.
absolute uncertainty: = 1.4
Mg/ml.
Incorrect
Method B: Dilute 1.000 mL up to 100 mL with a transfer pipet and volumetric flask.
absolute uncertainty: +
0.6
Incomect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 47 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



