Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter13: Acids And Bases
Section: Chapter Questions
Problem 58QAP: Consider citric acid, H3C6H5O7, added to many soft drinks. The equilibrium constants for its...
Related questions
Question
Answer both parts please :) thank you!
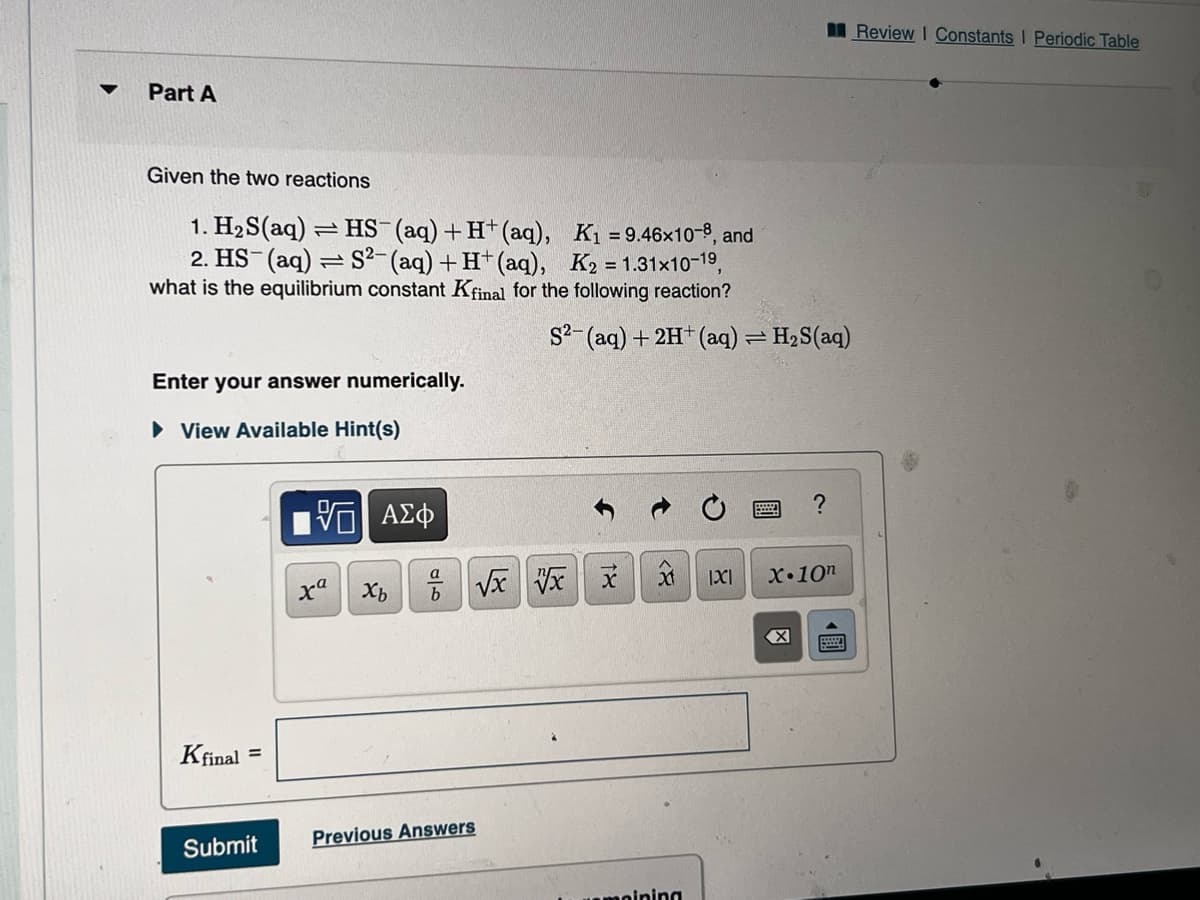
Transcribed Image Text:Part A
Given the two reactions
1. H₂S(aq) = HS- (aq) + H+ (aq), K₁ = 9.46x10-8, and
2. HS (aq) = S²- (aq) + H+ (aq), K2 = 1.31x10-1⁹,
what is the equilibrium constant Kfinal for the following reaction?
Enter your answer numerically.
► View Available Hint(s)
Kfinal =
Submit
G| ΑΣΦ
xa
Xb
a
b
S2- (aq) + 2H+ (aq) = H₂S(aq)
√x xx
Previous Answers
t
xd
noining
IXI
?
X.10n
Review I Constants I Periodic Table
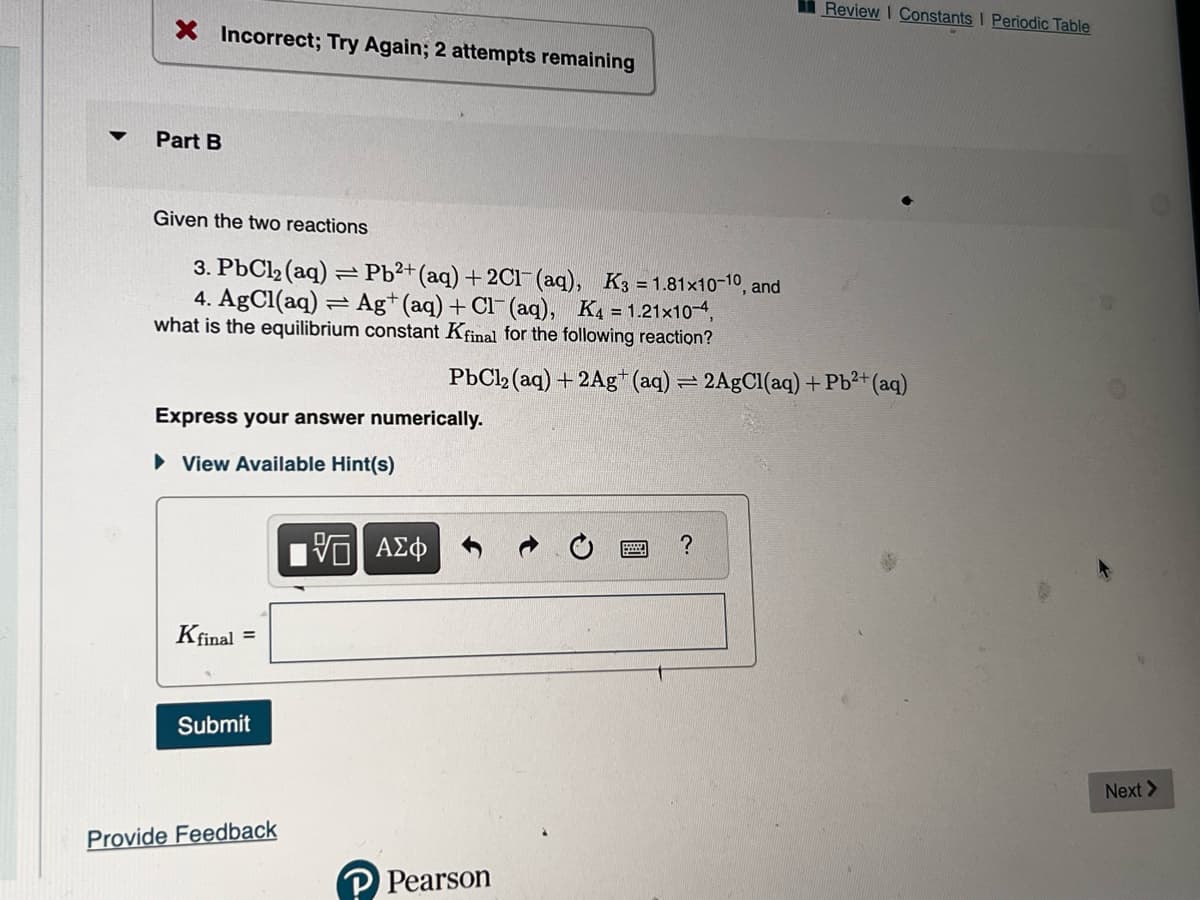
Transcribed Image Text:▼
X Incorrect; Try Again; 2 attempts remaining
Part B
Given the two reactions
3. PbCl₂ (aq) = Pb²+ (aq) + 2Cl(aq), K3=1.81x10-10, and
4. AgCl(aq) Ag+ (aq) + Cl(aq), K4 = 1.21x10-4,
what is the equilibrium constant Kfinal for the following reaction?
=
Express your answer numerically.
View Available Hint(s)
Kfinal =
Submit
Provide Feedback
ΑΣΦ
PbCl2 (aq) + 2Ag+ (aq) = 2AgCl(aq) + Pb²+ (aq)
Pearson
Review I Constants | Periodic Table
?
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning