Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest are: acetic acid (pK, = 4.8), and hydrogen bromide (pKa = -9). CH3 Br CH3 H-Br OH A B D acetic acid bromide acetate hydrogen bromide a) The stronger acid is HBr b) Its conjugate base is Br- c) The species that predominate at equilibrium are (two letters, e.g. ac) ad Submit Answer Retry Entire Group 9 more group attempts remaining
Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest are: acetic acid (pK, = 4.8), and hydrogen bromide (pKa = -9). CH3 Br CH3 H-Br OH A B D acetic acid bromide acetate hydrogen bromide a) The stronger acid is HBr b) Its conjugate base is Br- c) The species that predominate at equilibrium are (two letters, e.g. ac) ad Submit Answer Retry Entire Group 9 more group attempts remaining
Chapter20: Carboxylic Acids And Nitriles
Section20.4: Substituent Effects On Acidity
Problem 9P
Related questions
Question
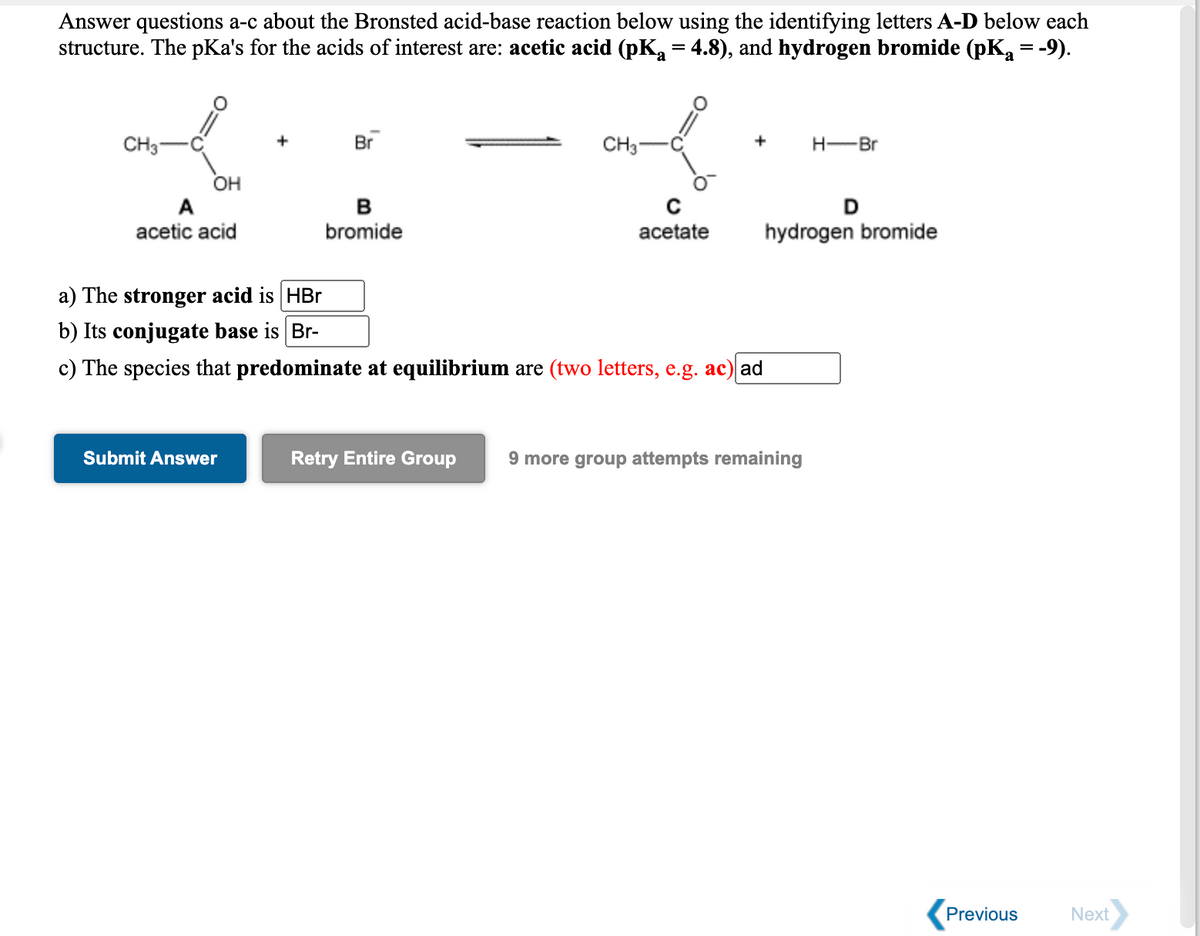
Transcribed Image Text:Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each
structure. The pKa's for the acids of interest are: acetic acid (pK = 4.8), and hydrogen bromide (pK = -9).
%3D
Br
CH3
CH3-
+
+
H-Br
OH
A
D
acetic acid
bromide
acetate
hydrogen bromide
a) The stronger acid is HBr
b) Its conjugate base is Br-
c) The species that predominate at equilibrium are (two letters, e.g. ac) ad
Submit Answer
Retry Entire Group
9 more group attempts remaining
Previous
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning