Answer the following question using the reaction coordinate diagram shown below. 280 240+ 200 160+ 120+ 80 + 40 Time How would the addition of a catalyst affect the reaction? A. Catalysts have no effect on actions. B. The rate of reaction will increase because potential energy of the reactants is increased. C. The rate of reaction will decrease because the activation energy is lowered. D. The rate of reaction will increase because the activation energy is decreased. Heat content (H) kilojoules
Answer the following question using the reaction coordinate diagram shown below. 280 240+ 200 160+ 120+ 80 + 40 Time How would the addition of a catalyst affect the reaction? A. Catalysts have no effect on actions. B. The rate of reaction will increase because potential energy of the reactants is increased. C. The rate of reaction will decrease because the activation energy is lowered. D. The rate of reaction will increase because the activation energy is decreased. Heat content (H) kilojoules
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.76E
Related questions
Question
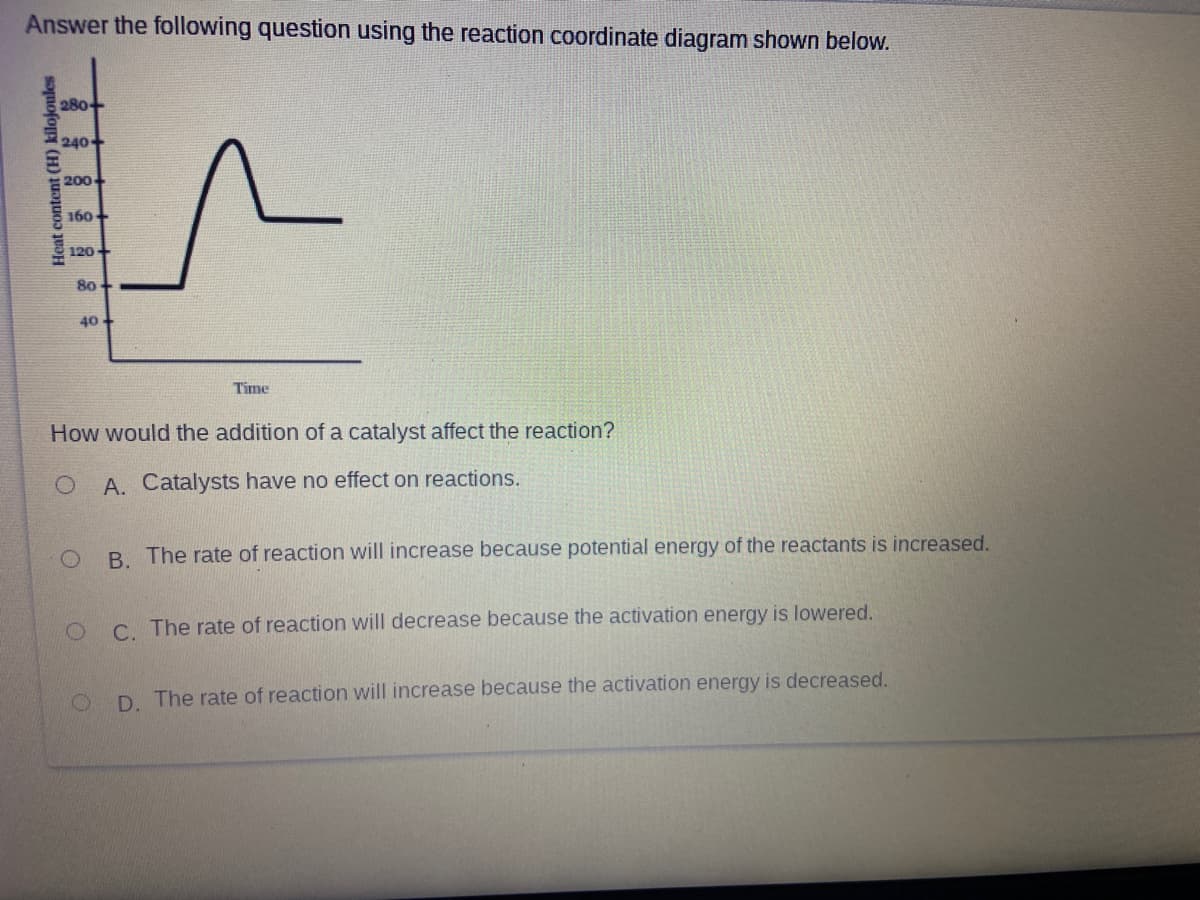
Transcribed Image Text:Answer the following question using the reaction coordinate diagram shown below.
280
240+
200
160
120+
80
40
Time
How would the addition of a catalyst affect the reaction?
A. Catalysts have no effect on reactions.
B. The rate of reaction will increase because potential energy of the reactants is increased.
C The rate of reaction will decrease because the activation energy is lowered.
D. The rate of reaction will increase because the activation energy is decreased.
Heat content (H) kilojoules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning