APOLLO 13 LUNAR MISSION ere are three steps needed for these calculations: 1) Convert grams → moles (using the molar mass of the known). 2) Convert moles of known (from step 1) → moles of unknown using the mole rati (from balanced chemical equation). 3) Convert moles of unknown (from step 2) → grams using molar mass of the unknown). 1 mol A Molar Mass A Molar Mass B 1 mol B mol B mol A Mass A Mol A Mol B Mass You are a NASA engineer. You are the chief engineer for the Apollo 13 mission to the moon. The astronauts are running out of oxygen and need to get rid of the excess carbon dioxide. You know that sodium hydroxide has been suggested as a means of removing carbon dioxide from the spacecraft cabin. The filter which they had been using is fully saturated and no longer works. You remember that the astronauts have a 3.000 g container of sodium hydroxide (NaOH) on the ship. You also know that sodium hydroxide can be used to remove carbon dioxide according to the following reaction: NaOH + co,- Na,co, + H,0 -How many grams co, can be removed from the ship with the 3,000 g of NaOH? - The astronauts have 2 days left before they land on earth. You know that the astronauts will emit roughly 1,000 grams of CO, each day. Is there enough sodium hydroxide in the cábin to cleanse the cabin air of the carbon dioxide, or are the astronauts doomed? Again be sure to show all your work,
APOLLO 13 LUNAR MISSION ere are three steps needed for these calculations: 1) Convert grams → moles (using the molar mass of the known). 2) Convert moles of known (from step 1) → moles of unknown using the mole rati (from balanced chemical equation). 3) Convert moles of unknown (from step 2) → grams using molar mass of the unknown). 1 mol A Molar Mass A Molar Mass B 1 mol B mol B mol A Mass A Mol A Mol B Mass You are a NASA engineer. You are the chief engineer for the Apollo 13 mission to the moon. The astronauts are running out of oxygen and need to get rid of the excess carbon dioxide. You know that sodium hydroxide has been suggested as a means of removing carbon dioxide from the spacecraft cabin. The filter which they had been using is fully saturated and no longer works. You remember that the astronauts have a 3.000 g container of sodium hydroxide (NaOH) on the ship. You also know that sodium hydroxide can be used to remove carbon dioxide according to the following reaction: NaOH + co,- Na,co, + H,0 -How many grams co, can be removed from the ship with the 3,000 g of NaOH? - The astronauts have 2 days left before they land on earth. You know that the astronauts will emit roughly 1,000 grams of CO, each day. Is there enough sodium hydroxide in the cábin to cleanse the cabin air of the carbon dioxide, or are the astronauts doomed? Again be sure to show all your work,
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.58E
Related questions
Question
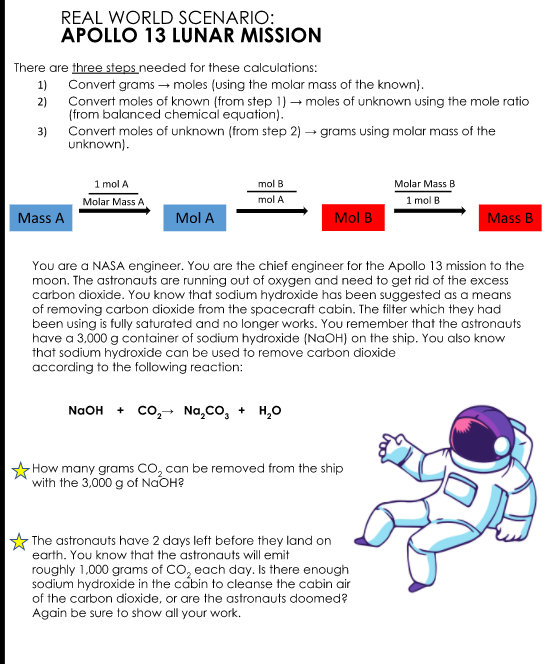
Transcribed Image Text:REAL WORLD SCENARIO:
APOLLO 13 LUNAR MİSSION
There are three steps needed for these calculations:
1)
Convert grams – moles (using the molar mass of the known).
Convert moles of known (from step 1) –→ moles of unknown using the mole ratio
(from balanced chemical equation).
2)
3)
Convert moles of unknown (from step 2) → grams using molar mass of the
unknown).
1 mol A
Molar Mass A
mol B
Molar Mass B
mol A
1 mol B
Mass A
Mol A
Mol B
Mass B
You are a NASA engineer. You are the chief engineer for the Apollo 13 mission to the
moon. The astronauts are running out of oxygen and need to get rid of the excess
carbon dioxide. You know that sodium hydroxide has been suggested as a means
of removing carbon dioxide from the spacecraft cabin. The filter which they had
been using is fully saturated and no longer works. You remember that the astronauts
have a 3.000 g container of sodium hydroxide (NAOH) on the ship. You also know
that sodium hydroxide can be used to remove carbon dioxide
according to the following reaction:
NaOH + Cо, Na,co, + H,0
-How many grams CO, can be removed from the ship
with the 3,000 g of NaOH?
- The astronauts have 2 days left before they land on
earth. You know that the astronauts will emit
roughly 1,000 grams of CO, each day. Is there enough
sodium hydroxide in the cabin to cleanse the cabin air
of the carbon dioxide, or are the astronauts doomed?
Again be sure to show all your work.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER