Apollo 13 Lunar Mission You are a NASA engineer. You are the chief engineer for the Apollo 13 mission to the moon The astronauts the are running out of oxygen and need to get rid excess carbon dioxide. You know that sodium hydrexide has been suggested as a means of removing carbon dioxide from the spacecraft cabin. The filter which they had been using is fully saturated and no longer works. You remember that the The astronauts have 2 days left before they land on astronauts have a 6.7 x 10 25 particles of sodium hydroxide on the ship in a container. You also know that sodium hydroxide can be used to remove carbon dioxide according to the following reaction earth. You know that there are three astronauts, and each astronaut emits roughly 500 g of carbon dioxide each day Is there enough sodium hydroxide in the cabin to cleanse the cabin air of the carbon dioxide, or are the astronauts doomed? co, Na,Co, + H,O NaOH
Apollo 13 Lunar Mission You are a NASA engineer. You are the chief engineer for the Apollo 13 mission to the moon The astronauts the are running out of oxygen and need to get rid excess carbon dioxide. You know that sodium hydrexide has been suggested as a means of removing carbon dioxide from the spacecraft cabin. The filter which they had been using is fully saturated and no longer works. You remember that the The astronauts have 2 days left before they land on astronauts have a 6.7 x 10 25 particles of sodium hydroxide on the ship in a container. You also know that sodium hydroxide can be used to remove carbon dioxide according to the following reaction earth. You know that there are three astronauts, and each astronaut emits roughly 500 g of carbon dioxide each day Is there enough sodium hydroxide in the cabin to cleanse the cabin air of the carbon dioxide, or are the astronauts doomed? co, Na,Co, + H,O NaOH
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.103P
Related questions
Question
I'm resending this question because the image on the last one was blurry. I wrote the prompt and steps and helpful conversions to figuring out the real world problem on my scratch paper, how do I solve for this problem?
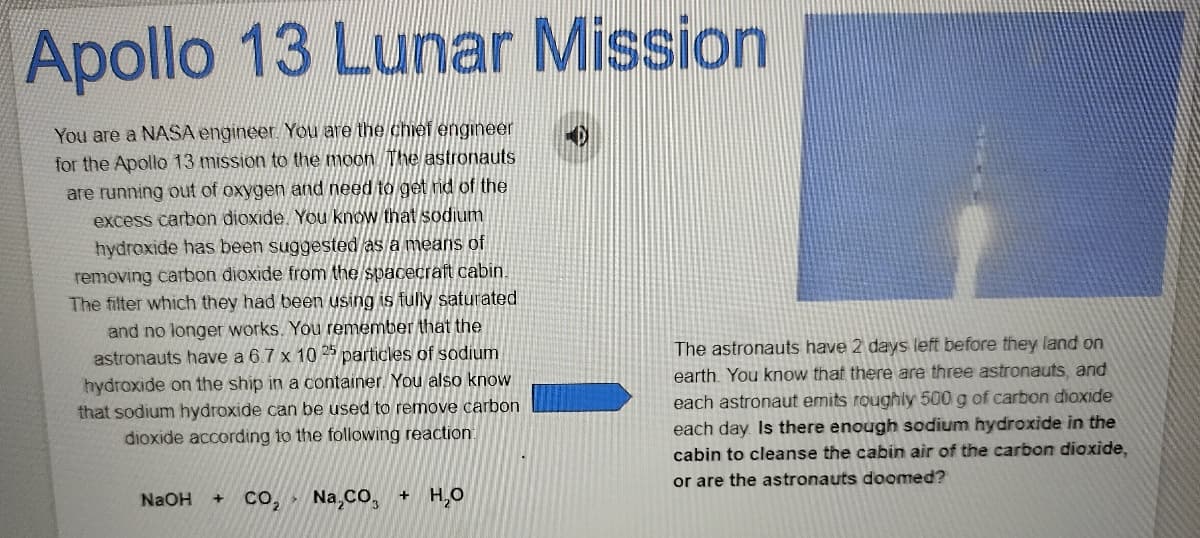
Transcribed Image Text:Apollo 13 Lunar Mission
You are a NASA engineer. You are the chief engineer
for the Apollo 13 mission to the moon The astronauts
are running out of oxygen and need to get rid of the
excess carbon dioxide. You know that sodium
hydroxide has been suggested as a means of
removing carbon dioxide from the spacecraft cabin.
The filter which they had been using is fully saturated
and no longer works. You remember that the
astronauts have a 6.7 x 10 25 particles of sodium
The astronauts have 2 days left before they land on
earth. You know that there are three astronauts, and
hydroxide on the ship in a container. You also know
that sodium hydroxide can be used to remove carbon
dioxide according to the following reaction.
each astronaut emits roughly 500 g of carbon dioxide
each day Is there enough sodium hydroxide in the
cabin to cleanse the cabin air of the carbon dioxide,
or are the astronauts doomed?
NaOH
Na,CO,
+ H,O

Transcribed Image Text:For Stoichiomery
chaverge
(Prompt:
with this real world Sceagsio:
a)1need
to Summarize the question/situation.
b) Storting point nding point
) what Conuersions will
you
need
d) Dimensional onalysis
e) How does your numerical onswer Solve this Scenorio?
Some helpful conversions:
molar mass: g
Avogadio's #!
1 moT
6.022x10
23
molecule s
tatoms)
1
moles
LA mole
raho : Coefficents between two Moiecules
By using Stoichiametry,
analysis 1 Can get
use this numerical onsuwes to refote the real wornd Scenario.
and information, units within the dimensional
correct numeric al analysis : And then 1 Con
Some Stipichmetry Steps 1 need to follow:
Conuest particCIeS grams moles (using Avogados number
1or the m0lar mass
2) Convert moles of the Known moles of unk nown using the mole ratio
from the balanced equation.
3 Convert the moles of the unknoun- particles/grams
using Avogrado's number or the moGr mass, respectivery)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax