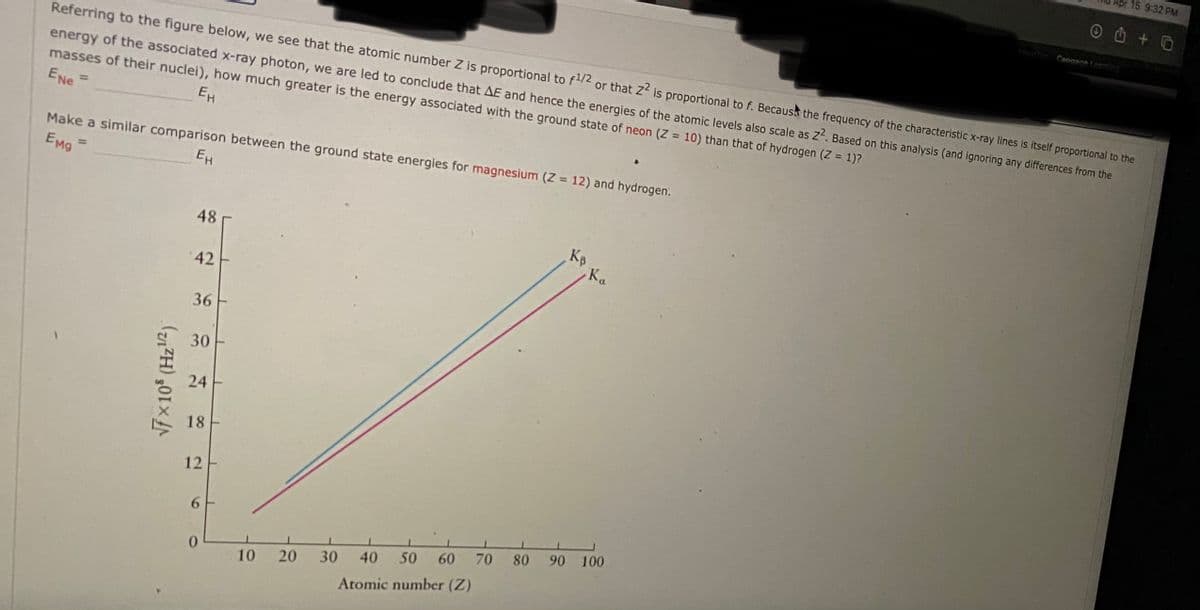
Apr 15 9:32 PM Cansne Referring to the figure below, we see that the atomic number Z is proportional to f2 or that z is proportional to f. Becaust the frequency of the characteristic x-ray lines is itself proportional to the energy of the associated x-ray photon, we are led to conclude that AE and hence the energies of the atomic levels also scale as z. Based on this analysis (and ignoring any differences from the masses of their nuclei), how much greater is the energy associated with the ground state of neon (Z = 10) than that of hydrogen (Z = 1)? ENe = EH Make a similar comparison between the ground state energies for magnesium (Z = 12) and hydrogen. EMg %3D 48 Kp Ka 42 36 30 24 18 12 6. 50 60 70 80 90 100 10 20 30 40 Atomic number (Z) V7 x10 (Hz2)
Apr 15 9:32 PM Cansne Referring to the figure below, we see that the atomic number Z is proportional to f2 or that z is proportional to f. Becaust the frequency of the characteristic x-ray lines is itself proportional to the energy of the associated x-ray photon, we are led to conclude that AE and hence the energies of the atomic levels also scale as z. Based on this analysis (and ignoring any differences from the masses of their nuclei), how much greater is the energy associated with the ground state of neon (Z = 10) than that of hydrogen (Z = 1)? ENe = EH Make a similar comparison between the ground state energies for magnesium (Z = 12) and hydrogen. EMg %3D 48 Kp Ka 42 36 30 24 18 12 6. 50 60 70 80 90 100 10 20 30 40 Atomic number (Z) V7 x10 (Hz2)
Chapter10: Atomic Physics
Section: Chapter Questions
Problem 18P
Related questions
Question
help please
Transcribed Image Text:Apr 15 9:32 PM
MindTan Canosna
Referring to the figure below, we see that the atomic number Z is proportional to f/2 or that Z is proportional to f. Becausa the frequency of the characteristic x-ray lines is itself proportional to the
energy of the associated x-ray photon, we are led to conclude that AE and hence the energies of the atomic levels also scale as Z. Based on this analysis (and ignoring any differences from the
masses of their nuclei), how much greater is the energy associated with the ground state of neon (Z = 10) than that of hydrogen (Z = 1)?
ENe
%3D
EH
Make a similar comparison between the ground state energies for magnesium (Z 12) and hydrogen.
EMg
%3D
%3D
EH
48
Kp
-Ka
42
36
30
24
18
12
70
80 90 100
40
50
60
10
20
30
Atomic number (Z)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill


Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

Horizons: Exploring the Universe (MindTap Course …
Physics
ISBN:
9781305960961
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning