Aqueous ethanol solutions are prepared by mixing measured weights of assayed ethanol and organic-free water. What is the molality of an aqueous solution that is 18.5 % ethanol, C2H5OH , by mass? Atomic Mass: C=12.01; H=1.008 ; O= 16.0 Express your answer in two decimal places. Do not write the unit. Add your answer
Aqueous ethanol solutions are prepared by mixing measured weights of assayed ethanol and organic-free water. What is the molality of an aqueous solution that is 18.5 % ethanol, C2H5OH , by mass? Atomic Mass: C=12.01; H=1.008 ; O= 16.0 Express your answer in two decimal places. Do not write the unit. Add your answer
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.44E
Related questions
Question
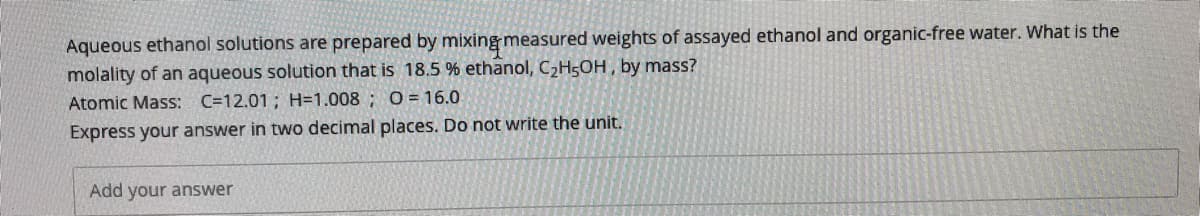
Transcribed Image Text:Aqueous ethanol solutions are prepared by mixing measured weights of assayed ethanol and organic-free water. What is the
molality of an aqueous solution that is 18.5 % ethanol, CH5OH , by mass?
Atomic Mass: C=12.01; H=1.008 ; O= 16.0
Express your answer in two decimal places. Do not write the unit.
Add your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co