aqueous solution of a purine triphosphate at a concentration of J. sodium dehydrate (molar mass 586 g/mol), give an absorbance of 1.014 with a light th of 1cm. Calculate the molar absorption coefficient. What would be the absorbance fa 10 uM solution, and what would be the percentage of light transmittance?
aqueous solution of a purine triphosphate at a concentration of J. sodium dehydrate (molar mass 586 g/mol), give an absorbance of 1.014 with a light th of 1cm. Calculate the molar absorption coefficient. What would be the absorbance fa 10 uM solution, and what would be the percentage of light transmittance?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter14: Applications Of Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 14.9QAP
Related questions
Question
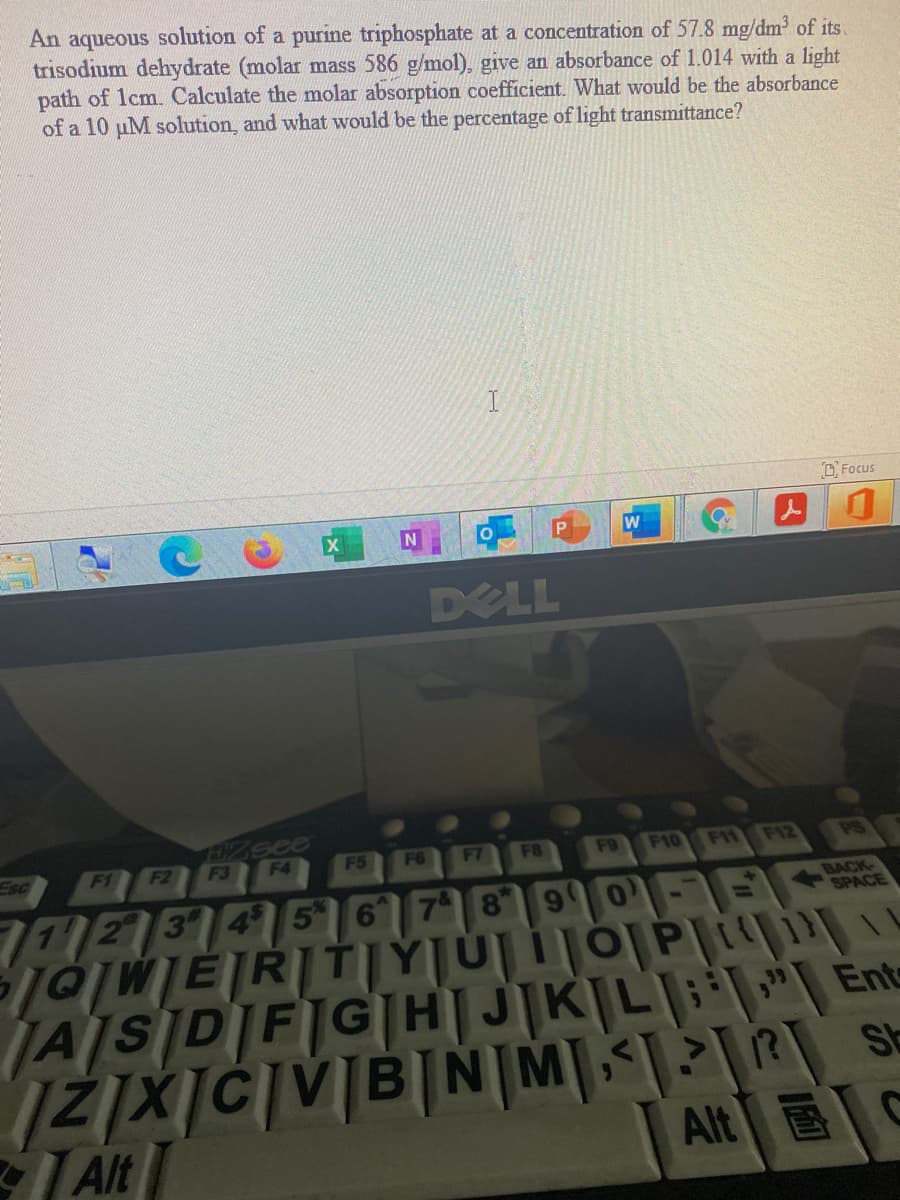
Transcribed Image Text:An aqueous solution of a purine triphosphate at a concentration of 57.8 mg/dm of its.
trisodium dehydrate (molar mass 586 g/mol), give an absorbance of 1.014 with a light
path of lcm. Calculate the molar absorption coefficient. What would be the absorbance
of a 10 uM solution, and what would be the percentage of light transmittance?
O Focus
DELL
Esc
F1
F2
F3
F4
F5
F6
F7
F8
F9
F10 F11 F12
PS
712 3456
8 9
BACK-
SPACE
JAISIDIFIGH|J|K|L|"| Ente
SE
Alt
Alt E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

