AS° for the reaction shown is equal to -667.8 (J/mol-K). Use the information given in the table to determine the standard entropy, S° (J/mol-K) for the substance C (J/mol-K). 3 A(s) + 3 B(g) → 3 C(s) + 2 D(g) Substance S°(J/mol-K) A(s) 160.0 B(g) 232.6 D(g) 254.0
AS° for the reaction shown is equal to -667.8 (J/mol-K). Use the information given in the table to determine the standard entropy, S° (J/mol-K) for the substance C (J/mol-K). 3 A(s) + 3 B(g) → 3 C(s) + 2 D(g) Substance S°(J/mol-K) A(s) 160.0 B(g) 232.6 D(g) 254.0
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.47PAE: The reaction CO2(g)+H2(g)CO(g)+H2O(g) is not spontaneous at room temperature but becomes spontaneous...
Related questions
Question

Transcribed Image Text:AS° for the reaction shown is equal to -667.8 (J/mol-K). Use the information given
in the table to determine the standard entropy, S° (J/mol-K) for the substance C
(J/mol-K).
3 A(s) + 3 B(g) → 3 C(s) + 2 D(g)
Substance
S°(J/mol-K)
A(s)
160.0
B(g)
232.6
D(g)
254.0
Expert Solution
Step 1
Given,
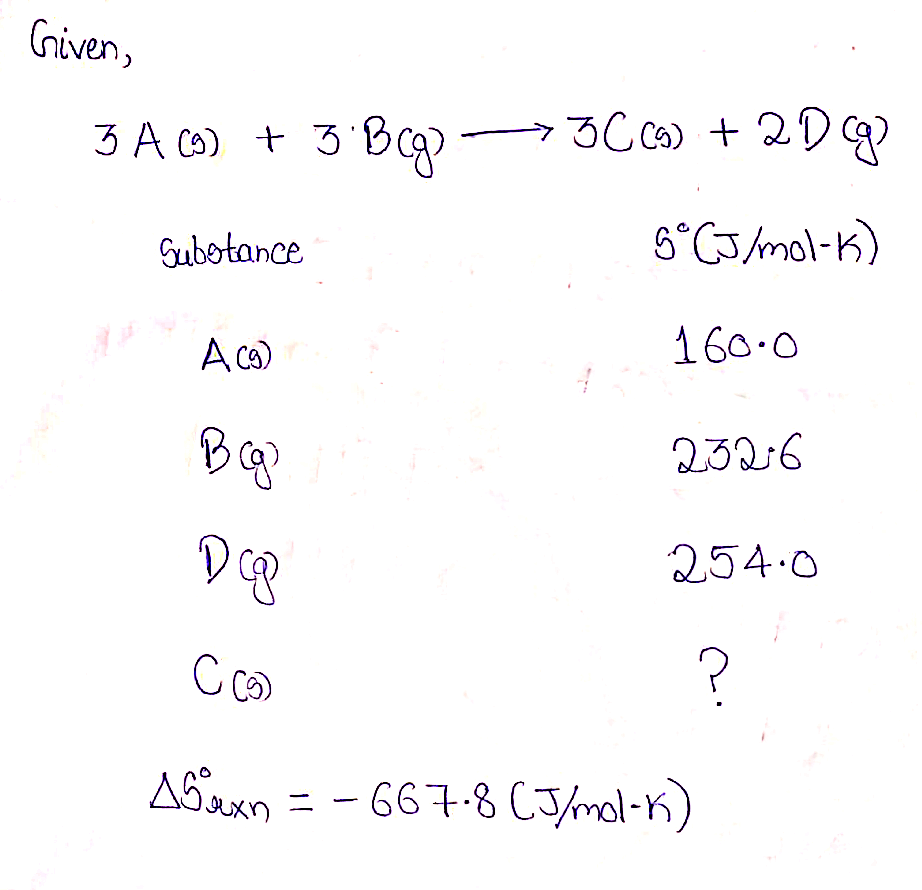
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning