As mentioned in the introduction, one type of bleach alternative uses hydrogen peroxide remove stains. The H₂O₂ in the product can be titrated with the MnO4 under acidic conditions by the reaction: 5 H₂O2(aq) + 2 MnO4 (aq) + 6 H(aq) 502(g) + 2 Mn2(aq) + 8 H₂O. If a 0.505g sample of a product containing H₂O₂ required 16.55mL of a 0.01158M MnO, solution to reach the end point, then what is the mass percent of hydrogen peroxide in this product
As mentioned in the introduction, one type of bleach alternative uses hydrogen peroxide remove stains. The H₂O₂ in the product can be titrated with the MnO4 under acidic conditions by the reaction: 5 H₂O2(aq) + 2 MnO4 (aq) + 6 H(aq) 502(g) + 2 Mn2(aq) + 8 H₂O. If a 0.505g sample of a product containing H₂O₂ required 16.55mL of a 0.01158M MnO, solution to reach the end point, then what is the mass percent of hydrogen peroxide in this product
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 58QAP: The iron content of hemoglobin is determined by destroying the hemoglobin molecule and producing...
Related questions
Question
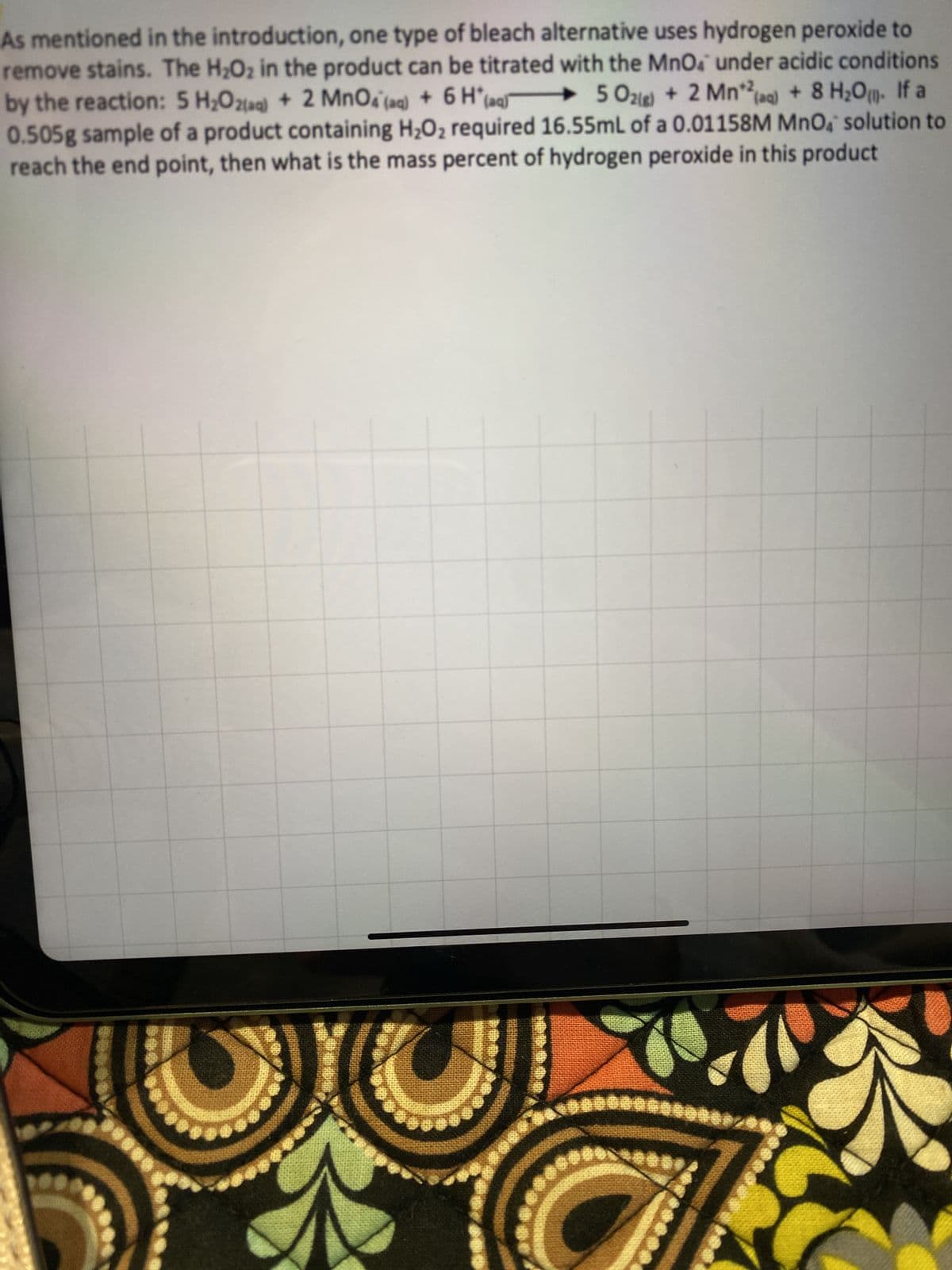
Transcribed Image Text:As mentioned in the introduction, one type of bleach alternative uses hydrogen peroxide to
remove stains. The H₂O₂ in the product can be titrated with the MnO4 under acidic conditions
by the reaction: 5 H₂O2(aq) + 2 MnO4 (aq) + 6 H(aq 502) + 2 Mn2(aq) + 8 H₂O. If a
0.505g sample of a product containing H₂O₂ required 16.55mL of a 0.01158M MnO solution to
reach the end point, then what is the mass percent of hydrogen peroxide in this product
*************
0000 40000
**
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning