Assign Oxidation States to All Elements: 0 Mg(s) + 0₂ (8) 2-2 Cuo + 0 H₂ (9) Fe₂O3(s) + 3 CO +1+5-2 AgNO3(aq) 0 MgO (s) Li (5) 0 → Cu (s) + +1 -2 H₂O) +1 +5-2 → LINO3(aq) +4-2 0 single → 3 CO₂ (4+ 2 Fe (s) displacement (8) + Equation 0 Ag (s) combustion synthesis reaction single displacement reaction Singe displacement reaction OXIDIZED Element HALF REACTION: HALF REACTION: HALF REACTION: HALF REACTION: REDUCED Element HALF REACTION: HALF REACTION: HALF REACTION: HALF REACTION: ONDIZIO 02 Cuo Fe203 (s) AgNO3 (aq) Mg H2 3 CO (g) Li (s)
Assign Oxidation States to All Elements: 0 Mg(s) + 0₂ (8) 2-2 Cuo + 0 H₂ (9) Fe₂O3(s) + 3 CO +1+5-2 AgNO3(aq) 0 MgO (s) Li (5) 0 → Cu (s) + +1 -2 H₂O) +1 +5-2 → LINO3(aq) +4-2 0 single → 3 CO₂ (4+ 2 Fe (s) displacement (8) + Equation 0 Ag (s) combustion synthesis reaction single displacement reaction Singe displacement reaction OXIDIZED Element HALF REACTION: HALF REACTION: HALF REACTION: HALF REACTION: REDUCED Element HALF REACTION: HALF REACTION: HALF REACTION: HALF REACTION: ONDIZIO 02 Cuo Fe203 (s) AgNO3 (aq) Mg H2 3 CO (g) Li (s)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.60QE
Related questions
Question
100%
Use the example for half reactions
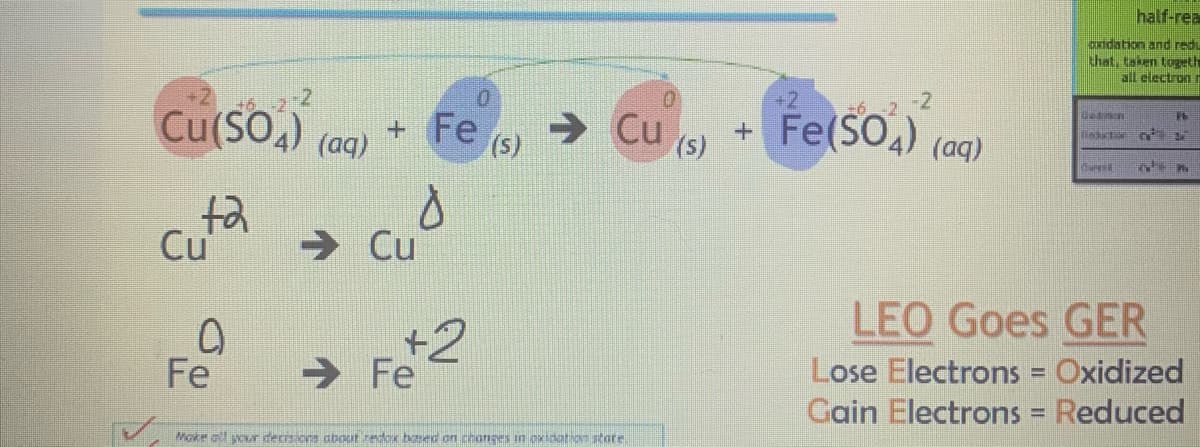
Transcribed Image Text:Cu(SO4) (aq) +
+2
Cu
0
Fe
→ Cu
Fe
→ Fe
d
+2
(S)
Cu
Moke all your decisions about redox based on changes in oxidation state.
(s)
+2
Fe(50₂) (aq)
+ (SO4)
half-rea
oundation and redu
that, taken togethe
all electron r
de doman
BladKEN
H
Owerk
M
LEO Goes GER
F6
42
P
Lose Electrons
Oxidized
Gain Electrons = Reduced
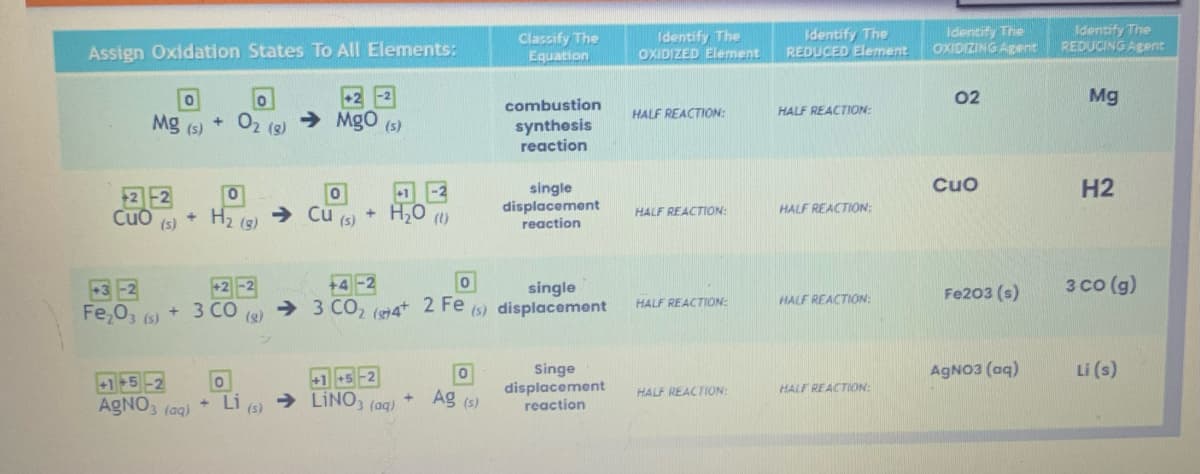
Transcribed Image Text:Assign Oxidation States To All Elements:
0
Mg (s)
0
22
Cuo(s) + H₂ (8)
0
02 (8)
Fe₂O3 (s) + 3 CO (2)
+1+5-2 0
AgNO3(aq)
+
Li (s)
→ MgO (s)
0
Cu (s)
+1 -2
H₂O
(1)
+1 +5-2
→ LINO3(aq)
Classify The
Equation
0
+ Ag (s)
combustion
synthesis
reaction
+4-2
0
single
→ 3 CO₂ (4+ 2 Fe (s) displacement
single
displacement
reaction
Singe
displacement
reaction
Identify The
OXIDIZED Element
HALF REACTION:
HALF REACTION:
HALF REACTION:
HALF REACTION:
Identify The
REDUCED Element
HALF REACTION:
HALF REACTION:
HALF REACTION:
HALF REACTION:
Identify The
OXIDIZING Agent
02
Cuo
Fe203 (s)
AgNO3(aq)
Identify The
REDUCING Agent
Mg
H2
3 CO (g)
Li (s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning