For each of the following valence electron configurations of a homonuclear diatomic molecule or molecular ion, identify the element X, Q, or Z and determine the total bond order. (a) X2:(0g2s)*(ơi2s)?(0g2p.)° (Tn2p)* (1Tg2p)* (b) Qi:(02;) (0i2:)} (7Tn2p)* (02p.)" (c) Zz:(0g2,)*(0i2s)?(0g2p.)*(Tu2p)*(12p)³
For each of the following valence electron configurations of a homonuclear diatomic molecule or molecular ion, identify the element X, Q, or Z and determine the total bond order. (a) X2:(0g2s)*(ơi2s)?(0g2p.)° (Tn2p)* (1Tg2p)* (b) Qi:(02;) (0i2:)} (7Tn2p)* (02p.)" (c) Zz:(0g2,)*(0i2s)?(0g2p.)*(Tu2p)*(12p)³
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter4: Molecular Structure And Orbitals
Section: Chapter Questions
Problem 2ALQ: Explain the difference between the and MOs for homonuclear diatomic molecules. How are bonding and...
Related questions
Question
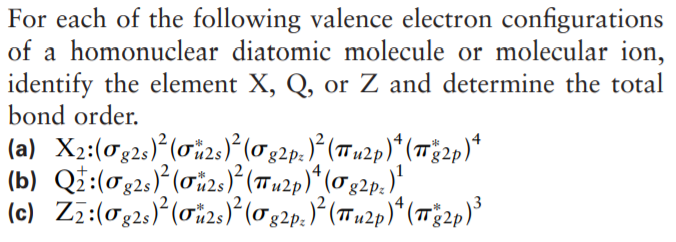
Transcribed Image Text:For each of the following valence electron configurations
of a homonuclear diatomic molecule or molecular ion,
identify the element X, Q, or Z and determine the total
bond order.
(a) X2:(0g2s)*(ơi2s)?(0g2p.)° (Tn2p)* (1Tg2p)*
(b) Qi:(02;) (0i2:)} (7Tn2p)* (02p.)"
(c) Zz:(0g2,)*(0i2s)?(0g2p.)*(Tu2p)*(12p)³
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning