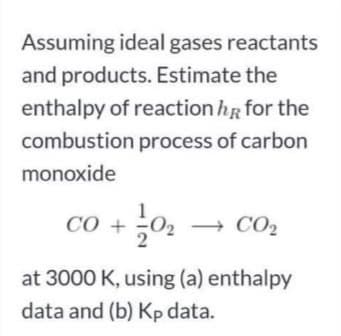
Assuming ideal gases reactants and products. Estimate the enthalpy of reaction hR for the combustion process of carbon monoxide CO + 02 + CO2 at 3000 K, using (a) enthalpy data and (b) Kp data.
Q: When a Tootsie Roll is burned in a calorimeter at 298 K and constant pressure, the resulting…
A: In a calorimeter, at constant pressure, the equations of enthalpy are,…
Q: The standard enthalpies of formation, at 25.00 oC, of methanol (CH4O(l)), water (H2O(l)), and carbon…
A: The heat energy gets measured in J/K and not available for performing useful work is basically…
Q: Sodium hydroxide reacts with a monoprotic weak acid HA according to the following balanced chemical…
A: Given: Volume of NaOH = 77 mL = 0.077 L (Since 1 L = 1000…
Q: Assuming that neither standard enthalpy changes of formations (∆Hof) nor standard molar entropies…
A: First, we shall estimate the enthalpy change of the reaction:…
Q: Benzoic acid of mass 1.40 g is reacted with oxygen in a constant volume calorimeter to form H2O(l)…
A: First, let's calculate the number of moles of benzoic acid combusted. Number of moles combusted=Mass…
Q: E2C.8(b) Estimate AH®(750 K) for the reaction N,(g) + H,(g) → NH,(g) from the listed value of the…
A:
Q: The standard enthalpy of combustion, A H; , of graphite is -393.51 kJ mol1. Calculate the change in…
A:
Q: For the reaction Cr(C6H6)2(s) → Cr(s) + 2 C6H6(g), ΔrU⦵(583 K) = +8.0 kJ mol−1. Find the…
A: Given: The reaction is as follows, delta Uorxn = + 8.0 kJ/mol Temperature = 583 K
Q: Calculate the final temperature and the change in enthalpy when 765 J of energy is transferred as…
A: The solution is given below:
Q: The standard enthalpy of combustion, A H: , of graphite is -393.51 kJ mol1. Calculate the change in…
A: Answer- Data given- standard enthalpy of combustion of graphite is -393.51 kaj mol-1. Kindly refer…
Q: Consider a process in which an ideal gas changes from state 1 to state 2 in such a way that its…
A: State functions: These functions depend only on the state and not on the path it underwent to reach…
Q: The first and second ionization enthalpies of calcium.Ca, are 596 kJ mol-1 and 1145 kJ mol-1…
A: Ionization enthalpy is defined as the minimum amount of energy required to remove the electron from…
Q: Using the standard heats of formation and the absolute entropies, calculate AG° (in units of kJ) for…
A: ∆G°= -2108KJ.
Q: Calculate the standard enthalpy of formation of phenol, C 6H50H, at 298.15 K given that, at this…
A: Change of enthalpy of reaction is difference between total enthalpy of products and total enthalpy…
Q: The standard enthalpy of combustion, A H:, of graphite is -393.51 kJ mol1. Calculate the change in…
A: Given, Standard enthalpy of combustion of graphite = ΔH0c = -393.51 KJ/mole Calculate the standard…
Q: Calculate the standard enthalpy of formation of phenol, C 6H5OH, at 298.15 K given that, at this…
A: We are given the reaction 2C6H5OH + 15O2 → 12CO2 + 6H2O ΔFC6H5OH = –165.0 KJ/mol ΔFH2O = –…
Q: What is AG (in kJ) for 2 SO2(g) + O2(g) 2 SO3(g) at 700 K, under standard conditions of 1 bar…
A: Given :- 2SO2(g) + O2(g) → 2SO3(g) K at 700 K = 3.0 × 104 To calculate :- ∆G (in kJ)
Q: Enthalpy of Chemical Reactions As stated earlier, the change in internal energy is related to the…
A:
Q: For the combustion of 1.00 mol of toluene, C7H8 , at 25 °C and 1.00 bar, calculate the work that…
A:
Q: The heat of combustion of glucose, C6H12O6, is -673Kcal at 298k. Calculate the standard enthalpy of…
A:
Q: Calculate the standard internal energy of formation of urea, (NH2)2CO, if its standard enthalpy of…
A:
Q: The standard enthalpy of combustion, A H , of graphite is -393.51 kJ mol-1. Calculate the change in…
A: Given : Enthalpy of combustion of graphite = -393.51 KJ/mol
Q: Work is done in this reaction when the H2 gas expands out of the test tube and pushes the atmosphere…
A: Explanation When H2 gas expands and pushes the atmosphere outside that is work is done by the gas…
Q: Imagine that 2.00 mol argon, confined by a moveable, fric- tionless piston in a cylinder at a…
A: Hello. Since your question has multiple sub-parts, we will solve the first three sub-parts for you.…
Q: What is the difference between internal energy and enthalpy? Under what conditions will they become…
A: Answer of this question :- Enthalpy :- it is the heat energy that is being absorbed or evolved…
Q: Calculate the difference, ΔH-ΔE=Δ(PV) for the combustion reaction of 1 mole of methane.
A:
Q: The standard enthalpy of combustion of propane, C3Hg, is -2.220 x 103 kJ mol-1 at 400 K. Use the…
A: Given : ∆H° for combustion of methane at 400 K = -2.220 x 103 KJ/mol T1 = 400 K T2 = 600 K
Q: P3C.6 Use values of enthalpies of formation, standard entropies, and standard heat capacities…
A:
Q: The standard enthalpy of formation of H2O(l) at 298 K is -285.8 kJ/mol.Calculate the change in…
A: Given: Standard enthalpy of formation of H2O(l) is = -285.8 kJ/mol Standard enthalpy of formation of…
Q: Calculate the enthalpy for the reaction of nitrogen dioxide gas with liquid water to make aqueous…
A:
Q: Hydrogen, a potential future fuel, can be produced from carbon (from coal) and steam by the…
A:
Q: What is the standard enthalpy of formation of the decomposition of one mole of liquid sulfuric acid…
A: Balanced chemical equation for decomposition of Sulfuric acid: 2H2SO4(l) → 2H2O(g) + O2(g) + 2SO2(g)…
Q: Benzoic acid of mass 1.35 g is reacted with oxygen in a constant volume calorimeter to form H20(1)…
A: The calorimeter constant can be calculated as shown in the following steps
Q: The standard enthalpy of combustion, AH,of graphite is -393.51 kJ mol 1. Calculate the change in…
A:
Q: The standard enthalpy of formation of gaseous H20 at 298 K is -241.82 kJ/mol. V heat capacities at…
A: Given reaction is H2 (g) + 1/2 O2 (g) --> H2O (g) molar heat capacity…
Q: The standard enthalpy of combustion, A H; ,of graphite is -393.51 kJ moli. Calculate the change in…
A:
Q: Use bond enthalpies and mean bond enthalpies to estimate (a) the enthalpy of the anaerobic breakdown…
A: (a) The required reaction is given below. The structure of the reactant and product are given…
Q: for the reaction Cr(C6H6)2 (s) → Cr(s) + 2 C6H6 (g) ArU© (583 K) = + 8.0 kJmol-1. find the…
A: The explanation is given below-
Q: What is AG (in kJ) for 2 SO2(g) + O2(g) – 2 SO3(g) at 700 K, under standard conditions of 1 bar…
A: Given :- 2SO2(g) + O2(g) →2SO3(g) K = 3.0 × 104 T = 700 K To calculate :- ∆G (in kJ)
Q: Consider
A: New Reaction equation is : 3A + 3/2B ------> 3C Then, it's energy also multiple by 3/2 fector…
Q: The standard enthalpy of combustion, A H; , of graphite is -393.51 kJ mol-1. Calculate the change in…
A: Internal energy is defined as the energy contained by a thermodynamic system.
Q: of water in the jacket. Put in the reaction chamber of the calorimeter 4 liters of water and 2…
A: We know from the caloriemetric process---- q = m × C × ∆T where C is the heat capacity and m is the…
Q: Use bond enthalpies and mean bond enthalpies to estimate (a) the enthalpy of the anaerobic breakdown…
A: The combustion of glucose in presence of oxygen will produce carbon dioxide and water molecules.
Q: What is the standard enthalpy for a reaction (J/mol) for which the equilibrium constant is 2 times…
A:
Q: Calculate the standard enthalpy change for the reaction 2C8H18(l) + 21O2(g) → 8CO(g) + 8CO2(g) +…
A:
Q: Calculate the heat of reaction at 500 K for the combustion of methane, CH4(g) + 2O2(g) → CO2(g) +…
A: (∆H°)reaction is the difference of ∆H of products to the ∆H of reactants.
Q: molar enthalpy of formation of H2O(l) from its elements in their standard states (25 oC and 100…
A: The standard molar enthalpy of formation of a compound is the change in enthalpy during the…
Step by step
Solved in 3 steps

- Estimate the enthalpy of reaction h̄R for the dissociation process CO2 ⇌ CO + 1⁄2 O2 at 2200 K, using (a) enthalpy data and (b) KP data.calculate the standard enthalpy for the reaction 2no2 -> n2o4 at 65 degree celcuidThe first and second ionization enthalpies of calcium.Ca, are 596 kJ mol-1 and 1145 kJ mol-1 respectively at 25 °c.Calculate the standard enthalpy change for the processCa(g) → Ca2+(g) + 2e- (g) at this temperature.
- Assuming that neither standard enthalpy changes of formations (∆Hof) nor standard molar entropies (So) depend upon temperature, estimate using the Table of Thermodynamic Data : (a) the standard Gibbs free energy change for the reaction that forms rhombic sulfur at 600 K, and (b) the temperature (in oC) at which reaction will stop formation of products: 2H2S(g) + SO2(g) → 3S(rhombic, s) + 2H2O(g) Round off your answers to the nearest integer. Report the temperature in oC. and enter them with correct units: (a)∆Gorxn = (b) T =From the data in Table 2C.4 of the Resource section, calculate ΔrH⦵ and ΔrU⦵ at (i) 298 K, (ii) 478 K for the reaction C(graphite) + H2O(g) → CO(g) + H2(g). Assume all heat capacities to be constant over the temperature range of interest.Calculate the change in S when one mole of water is heated from 263 to 283 K given the molar capacities inJ.K-1 , Cp(ice) = 2.09 + 0.126T, Cp(water)=75.3, and change in Hm=6000Jmol-1
- a.) Slove for the Gibbs energy of formation for a generic compound AB2 in kJ/mol given the following thermodynamic data. ΔfH∘kJ/mol Sf∘ J/mol K A(l) 0 75 B2(g) 0 220 AB2(s) -200 140 b. According the result from a, will the formation of AB2(s) occur under standard state conditions? Why or why not?Calculate the change in Gibbs energy for each of the sets of ΔrH∘, ΔrS∘, and T.The entropy of reaction at T= 350.15K and standard pressure.
- The standard Gibbs energy of formation of rhombic sulfur is zero, and that of monoclinic sulfur is +0.33 kJ mol−1 at 25 °C. The standard molar entropy of rhombic sulfur is 31.80 J K−1 mol−1, and that of monoclinic sulfur is 32.6 J K−1 mol−1. At what temperature will the transition occur at 1 bar? _______ K. 3 sig. fig.The standard Gibbs free energy change is 2.60 kJ/mol for the reaction at 25.0oC: H2(g) + I2(g) →← 2HI(g); ∆Gorxn = + 2.60 kJ/mol The reaction starts with equal molar amounts of H2(g) and I2(g) in a previously evacuated, constant volume vessel. What is the equilibrium mole fraction of HI? Enter your answers with correct units and significant figures.Coiled coils are protein domains that lead to the formation of multimers (dimers,trimers, tetramers, etc.). They are found in a wide range of proteins. Here we willconsider the trimerization reaction. We may write the chemical equilibrium as3 M(aq) ⇄ T(aq)where M and T represent, respectively, the monomer and trimer species. Given that∆rG0 = −25.00 kJ mol−1 at 37 0C (with the standard state the normal one for reactionsin solution, namely c0 = 1.0 mol dm−3):A) What is the equilibrium constant for the formation of the trimer at 37 0C? B) Suppose that in a particular experiment at 37.00 0C the initial concentrations ofthe monomer if [M]0 = 2.00×10−2 µM and the initial concentration of the trimeris also [T]0 = 2.00 × 10−2 µM. What is ∆rG for the formation of the trimer atthe specified temperature? C) For the conditions given in part (B) will trimers spontaneously convert to monomers?Give a very short justification of your answer. D) Suppose that ∆rH0 and ∆rS0 are independent of the…

