At 25 °C, a titration of 15.00 mL of a 0.0200 M AGNO, solution with a 0.0100 M NaI solution is conducted within the cell SCE || titration solution | Ag(s) For the cell as written, what is the potential after the addition of each volume of Nal solution? The reduction potential for the saturated calomel electrode is E = 0.241 V. The standard reduction potential for the reaction Ag+ + e → Ag(s) is E° = 0.79993 V. The solubility constant of AgI is Kp = 8.3 x 10-17. 0.500 mL E = V
At 25 °C, a titration of 15.00 mL of a 0.0200 M AGNO, solution with a 0.0100 M NaI solution is conducted within the cell SCE || titration solution | Ag(s) For the cell as written, what is the potential after the addition of each volume of Nal solution? The reduction potential for the saturated calomel electrode is E = 0.241 V. The standard reduction potential for the reaction Ag+ + e → Ag(s) is E° = 0.79993 V. The solubility constant of AgI is Kp = 8.3 x 10-17. 0.500 mL E = V
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 115AE: The saturated calomel electrode. abbreviated SCE. is often used as a reference electrode in making...
Related questions
Question
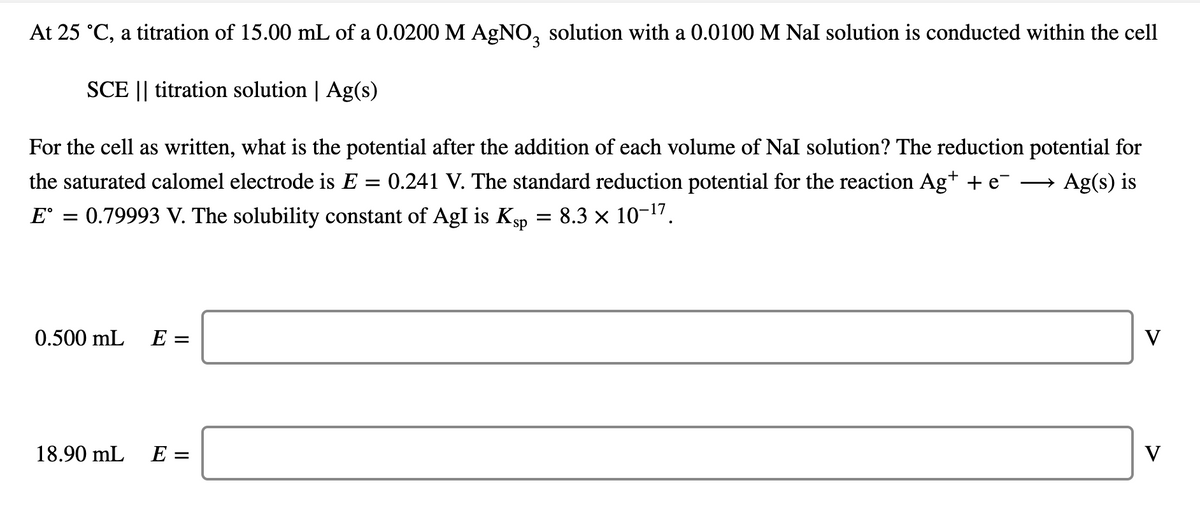
Transcribed Image Text:At 25 °C, a titration of 15.00 mL of a 0.0200 M AGNO, solution with a 0.0100 M Nal solution is conducted within the cell
SCE || titration solution | Ag(s)
For the cell as written, what is the potential after the addition of each volume of Nal solution? The reduction potential for
the saturated calomel electrode is E = 0.241 V. The standard reduction potential for the reaction Ag+ + e-
E° = 0.79993 V. The solubility constant of AgI is Kgp = 8.3 × 10-1".
Ag(s) is
>
0.500 mL E =
V
18.90 mL
E =
V

Transcribed Image Text:30.00 mL
E =
V
39.80 mL
E =
V
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning